†Corresponding author. E-mail: xmqin@shnu.edu.cn
‡Corresponding author. E-mail: ywlong@iphy.ac.cn
*Project supported by the National Basic Research Program of China (Grant No. 2014CB921500) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB07030300).
A high-quality SrFe0.8Co0.2O3 single crystal is prepared by combining floating-zone and high-pressure treatment methods. Its Magnetocaloric effect is investigated by magnetic measurements. A paramagnetism-to-ferromagnetism transition is found at about 270 K and this transition is a second-order one in nature as confirmed by Arrott plots. The saturated moment obtained at 2 K and 7 T is 3.63 μB/f.u. The maximal value of magnetic entropy change measured at 5 T is about 4.0 J·kg−1·K−1. The full wide at half maximum for a magnetic entropy change peak observed in SrFe0.8Co0.2O3 is considerably large. As a consequence, the relative cooling power value of SrFe0.8Co0.2O3 obtained at 5 T is 331 J/kg, which is greatly higher than those observed in other perovskite oxides. The present work therefore provides a promising candidate for magnetic refrigeration near room temperature.
The magneto-thermodynamic phenomenon known as the magnetocaloric effect (MCE) was first discovered by Warburg in 1881.[1] Its application in magnetic refrigeration provides a unique way to realize refrigeration from ultralow to room temperature (RT).[2– 4] The MCE refers to the temperature change in response to the variation in the external magnetic field. For a paramagnetic solid near absolute zero or a ferromagnetic material near its Curie temperature (TC), when the applied magnetic field increases in an adiabatic way, the magnetic part of the total entropy is released to the lattice, resulting in temperature rising in the material. In the reverse demagnetizing process, the zero field magnetic entropy of the material or refrigerant is restored from the lattice part, leading to the falling of the refrigerant temperature.[5, 6]
Compared with conventional gas compression refrigeration, the ozone depletion substance and compressor are not needed in the magnetic refrigeration. Moreover, the efficiency of magnetic refrigeration can be as high as 30– 60% of Carnot cycle, which is much higher than that of the gas compression refrigeration (5– 10%).[7] Therefore, magnetic refrigeration is very environmentally friendly and energy saving as well as noiseless and stable as compared with gas compression refrigeration.[4, 8– 10] At present, much attention has been paid to searching for room-temperature MCE materials such as Gd, [11] MnAs, [12] Gd5Si2Ge2, [13] MnFeP0.45As0.55, [14] etc, owing to the potential environmental and economic benefits.[15, 16] However, these materials usually are expensive, poisonous and have hystereses in magnetic and thermal cycles arising from the fist-order ferromagnetic transition. Hysteresis can depress the efficiency of magnetic refrigeration. By comparison, the second-order MCE materials have no magnetic and thermal hysteresis and usually have comparable or even larger refrigerant capacity (RC), although they sometimes exhibit relatively low values of magnetic entropy change (Δ SM). It is therefore very desirable to search for MEC materials with second-order ferromagnetic transition and considerable RC near RT.[6]
ABO3-type perovskite is a kind of very important functional materials due to its versatile crystal structures and physical properties.[16, 17] The MCE was studied in a few of perovskite compounds such as BaFeO3, [19] La1− xCaxMnO3, [20, 21] Ba2Fe1+ xMo1− xO6, [22] etc. As for the solid solution of SrFe1− xCoxO3 perovskite, with increasing Co content (x), the ground state magnetism changes from antiferromagnetism (x ≤ 0.05) through cluster glass to ferromagnetism (x > 0.15) with high ferromagnetic Curie temperatures between 245 and 337 K.[23] The tunable curie temperature near RT makes this solid solution an interesting candidate for MCE study. Actually, the MCE of SrFe0.5Co0.5O3 polycrystalline sample was investigated by Yin et al., which exhibits a second-order magnetic transition around 330 K.[24] The Δ SM is an important parameter to qualify MCE for a refrigerant material. A large saturated moment is favorable to enhancing the value of Δ SM. In the SrFe1− xCoxO3 solid solution, the largest saturated moment emerges near x = 0.2.[23] Moreover, the value of saturated moment in this family is related to sample quality. For example, in SrCoO3 polycrystalline samples, the saturated moments obtained in experiments are between 1.0 and 1.8μ B/f.u., [25, 26] which is much less than that observed in the single-crystal sample (2.5μ B/f.u.).[23, 27] We thus prepare high-quality SrFe0.8Co0.2O3 single crystal and study the magnetocaloric effect in this paper.
Oxygen stoichiometric SrFe0.8Co0.2O3 single crystal was grown by a two-step method.[23, 27] Firstly, oxygen-deficient polycrystalline sample SrFe0.8Co0.2O2.5 with brownmillerite-type structure was prepared by a standard solid-state reaction method by using high-purity SrCO3, Fe2O3, and Co3O4 as starting materials. These reactants were thoroughly mixed and then sintered at 1373 K for 24 h in air. The product was ground again and pressed into a rod with 5 mm in diameter and 10 cm in length. This rod was treated at 1373 K for 12 h in Ar atmosphere. Single crystal of SrFe0.8Co0.2O2.5 was grown by a floating-zone method with using the prepared rod. Finally, the obtained SrFe0.8Co0.2O2.5 single crystal was treated in a cubic-anvil-type high pressure apparatus at 6.0 GPa and 1023 K for 30 min in the presence of KClO4 oxidant. Thermogravimetric analysis confirmed that the final product treated by high oxygen pressure is SrFe0.8Co0.2O3 with stoichiometric oxygen content. The crystal structure of SrFe0.8Co0.2O3 was checked by a Rigaku diffractometer with Cu-Kα radiation (40 kV, 300 mA).The quality of single crystal was identified by Laue diffraction. The magnetic data were measured by using a quantum design vibrating sample magnetometer. The temperature dependence of magnetic susceptibility was acquired at applied magnetic field H = 0.1 T in zero-field-cooled (ZFC) and field-cooled (FC) modes.
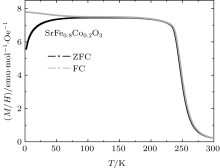
The as-prepared sample after high-oxygen-pressure treatment is proved to be a high-quality single crystal by Laue diffraction measurements. Figure 1 shows the x-ray powder diffraction pattern measured at room temperature. All the diffraction peaks can be well indexed in terms of a simple cubic perovskite structure with space group Pm-3m. The obtained lattice constant is a = 3.8480(1) Å , which is in agreement with previously reported result.[23] The temperature dependences of magnetic susceptibility measured at magnetic field H = 0.1 T in ZFC and FC modes are shown in Fig. 2. A ferromagnetic transition is observed at TC ≈ 270 K in the present SrFe0.8Co0.2O3 single crystal. No substantial hysteresis is found in the transition temperature region, implying that the magnetic transition is second-order in nature.
 | Fig. 1. Powder x-ray diffraction pattern and corresponding Miller indices of SrFe0.8Co0.2O3 single crystal. |
Figure 3 shows isothermal magnetization curves measured at several representative temperatures. At 300 K the magnetization does not show a linear dependence on magnetic field, suggesting the presence of some short-range ferromagnetic interactions above the Curie temperature. Actually, in the present SrFe0.8Co0.2O3 system, multiple ferromagnetic double-exchange interactions are possible to occur between Fe4+ and Co4+ ions as well as between Fe4+ /Co4+ ions and oxygen holes due to the strong p– d hybridizations.[26, 28] The saturated moment obtained at 2 K and 7 T is 3.63μ B/f.u., which is slightly smaller than the completely local spin moment (3.8μ B/f.u.) for the high-spin Fe4+ and intermediate-spin Co4+ ions in the metallic SrFe0.8Co0.2O3 as proposed before.[23] Note that the saturated moment of SrFe0.8Co0.2O3 is largest in the ferromagnetic region in SrFe1− xCoxO3 family. For example, the saturated moment observed in our sample is considerably larger than that of SrFe0.5Co0.5O3 (∼ 3.02μ B/f.u.), suggesting a greater MCE for the present SrFe0.8Co0.2O3.[24] Moreover, the coercive field at 2 K is only 200 Oe as shown in the inset of Fig. 3. With increasing temperature, the coercive field is reduced sharply. As an instance, almost no magnetic hysteresis is shown up above 200 K. This is favorable for the potential application for an MCE material.
 | Fig. 3. Isothermal magnetization curves of SrFe0.8Co0.2O3 measured at several representative temperatures. Inset shows the low-field magnetization curves to clarify the small coercive fields. |
To study the MCE of SrFe0.8Co0.2O3 single crystal, a series of isothermal magnetization curves is measured from 200 K to 300 K in steps of 2 K as shown in Fig. 4(a). On the basis of these measurements, the Arrott plots (H/M versus M2) are obtained to identify the nature of the ferromagnetic phase transition. According to Banerjee’ s criterion, [29] if all the Arrott plots have positive slopes, the magnetic transition should be second-order. On the other hand, if a negative slope emerges, then the magnetic order is first-order. As presented in Fig. 4(b), all the Arrott plots show positive features, revealing that the ferromagnetic phase transition occurring in SrFe0.8Co0.2O3 is second-order in nature, which is in agreement with magnetic susceptibility measurements mentioned above. The magnetic Curie temperature determined from the Arrott plots is about 274 K.
 | Fig. 4. (a) A series of isothermal magnetization curves of SrFe0.8Co0.2O3 measured between 200 and 300 K, (b) Arrott plot (H/M versus M2) constructed from the magnetization data. |
According to the thermodynamics theory, the Δ SM can be calculated by using the following function:

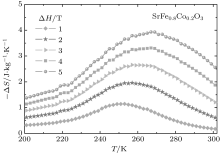
Here, Σ is the area between two MH curves measured at temperatures T1 and T2, respectively. The calculated − Δ SM(T) from the MH curves is shown in Fig. 5. The maximal value of − Δ SM(T) measured at 5 T in SrFe0.8Co0.2O3 is about 4.0 J· kg− 1· K− 1, which is comparable to that of SrFe0.5Co0.5O3. However, the full width at half maximum (TFWHM) for a − Δ SM(T) peak observed in the former is clearly larger than that in the latter, indicating an enhanced refrigerant capacity in the present SrFe0.8Co0.2O3 single crystal.
The refrigerant capacity and relative cooling power (RCP) are important parameters to evaluate magnetic cooling efficiency for a magnetocaloric material.[30– 35] They can respectively be defined by the formulas

Here, 
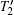
Oxygen stoichiometric SrFe0.8Co0.2O3 single crystal is grown by a two-step high-pressure single crystal growth method. Its magnetocaloric effect is investigated in detail by magnetic measurements. A paramagnetism-to-ferromagnetism transition is observed at about 270 K on the basis of the temperature dependence of magnetic susceptibility. No substantial thermal hysteresis is found in the transition temperature region. Isothermal magnetization curves each show a large saturated moment at 2 K and 7 T (3.63μ B/f.u.), and almost no magnetic hysteresis is found around the Curie temperature. All the Arrott plots show positive features, revealing that the ferromagnetic phase transition is second-order in nature. The maximal value of − Δ SM(T) measured at 5 T in SrFe0.8Co0.2O3 is about 4.0 J· kg− 1· K− 1, which is comparable to that of SrFe0.5Co0.5O3. However, the TFWHM for a − Δ SM(T) peak observed in the former is clearly larger than in the later. As a consequence, enhanced RC and RCP values are obtained in SrFe0.8Co0.2O3. These values (RC = 277 J/kg and RCP = 331 J/kg at 5 T) are considerably higher than those observed in most of other perovskite oxides, and even close to those of the well-studied giant magnetocaloric materials such as Gd5Si2Ge2 with a first-order ferromagnetic transition. On account of the wide working temperature, large − Δ SM and RC, as well as excluding rare elements, the present SrFe0.8Co0.2O3 provides a potential candidate for magnetic refrigeration near room temperature.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|