†Corresponding author. E-mail: zpwang@sdu.edu.cn
‡Corresponding author. E-mail: sunxun@sdu.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 51323002 and 51402173), the Independent Innovation Foundation of Shandong University, China (Grant Nos. IIFSDU and 2012JC016), the Program for New Century Excellent Talents in University, China (Grant No. NCET-10-0552), the Fund from the Key Laboratory of Neutron Physics, China Academy of Engineering Physics (Grant No. 2014BB07), and the Natural Science Foundation for Distinguished Young Scholar of Shandong Province, China (Grant No. JQ201218).
In this paper, the Raman gain coefficients of ammonium dihydrogen phosphate (ADP) and potassium dihydrogen phosphate (KDP) crystals are measured. By using a pump source of a 30-ps, 532-nm laser, the gain coefficients of ADP and KDP are 1.22 cm/GW, and 0.91 cm/GW, respectively. While for a 20-ps, 355-nm pump laser, the gain coefficients of these two crystals are similar, which are 1.95 cm/GW for ADP and 1.86 for KDP. The present results indicate that for ultra-violet frequency conversion, the problem of stimulated Raman scattering for ADP crystal will not be more serious than that for KDP crystal. Considering other advantages such the larger nonlinear optical coefficient, higher laser damage threshold, and lower noncritical phase-matching temperature, it can be anticipated that ADP will be a powerful competitor to KDP in large aperture, high energy third-harmonic generation or fourth-harmonic generation applications.
Potassium dihydrogen phosphate (KDP) crystal has been widely used as a nonlinear optical (NLO) material in inertial confinement fusion (ICF) equipment. For ultra-violet (UV) frequency conversion, the transverse stimulated Raman scattering (TSRS) is a critical limiting factor for enhancing output energy and conversion efficiency. In the past several decades, many researchers have focused on the Raman properties of KDP crystals.[1– 6] In fact, some ICF equipment, like the National Ignition Facility (NIF) of America, have used a partially deuterated KDP (DKDP) crystal to relieve the TSRS problem.[7]
In recent years, many advantages have been found in ammonium dihydrogen phosphate (ADP) crystal as compared with KDP crystal, such as larger nonlinear optical (NLO) coefficient, higher laser damage threshold, and bigger angular acceptance. Nevertheless, the stimulated Raman scattering (SRS) characteristics of ADP-type crystals must be evaluated first for practical applications. The spontaneous Raman scattering and SRS effect of ADP crystal have been reported before, [8, 9] and the pump wavelength is visible (532 nm) or near infrared (1064 nm). In the present paper, by comparing the SRS property at third-harmonic generation (THG) wavelength of Nd:YAG laser (355 nm), we find that the UV Raman gains are very close for ADP and KDP crystals, further confirming the application potential of ADP-type crystals in UV NLO fields.
Single crystals of ADP and KDP are grown from NH4H2PO4 and KH2PO4 solution using the rapid crystal growth method.[10] In the growth process, the velocity of temperature-reduction is maintained to be 0.1 ° C/day. The crystal rotated in the mode of “ forward-stop-backward” with a speed of about 77 rpm.[11] The pristine crystals are carefully cut along the a, b, and c axes, each with a size of 17 mm× 17 mm× 14 mm with a precision of 1′ , the specimens are then optically polished.
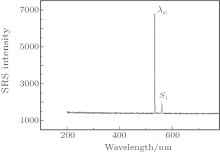
The experiments of spontaneous Raman scattering are conducted with a HR800 Raman spectrometer (France), the pump wavelength λ p is 633 nm, and the results are shown in Fig. 1. The information about the spectral line near 920 cm− 1 is also listed in Table 1. It attributes to the symmetrical vibration of [PO4] tetrahedron group. It can be seen that the line width Δ Ω R(ADP) = 28 cm− 1 and Δ Ω R(KDP) = 23 cm− 1. Compared with KDP, ADP exhibits a more intense and broader Raman line.
For steady state stimulated Raman scattering (SRS), the gain coefficient gSS is determined by the Raman properties of a nonlinear medium[12]
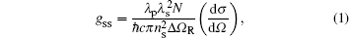
where N is the number of scattering centers (here, NADP = 0.944 × 1022 molecules/cm3, NKDP = 1.035 × 1022 molecules/cm3), λ p and λ s are the pump and Stokes wavelengths (λ p = 633 nm, λ s(ADP) = 730.14 nm, λ s(KDP) = 671.96 nm), nS is the refractive index at λ s (nS(ADP)= 1.5202 at 672.32 nm, nS(KDP) = 1.5060 at 671.96 nm) and d σ /dΩ is the Raman scattering cross section. Thus, referencing to Table 1 the steady state Raman gain coefficients of ADP and KDP crystals at 633-nm pump condition can be calculated, and the result of gSS(ADP) is 1.58 times larger than that of gSS(KDP). On the other hand, the value of the product (dσ /dΩ )(Δ Ω R)− 1 can be characterized as a peak intensity Σ peak in the measured spontaneous Raman scattering spectrum[12]

From Table 2 and Eq. (2), it follows that by using Eqs. (1) and (2), the result of gSS(ADP)/gSS(KDP) can be obtained to be 1.58.
by using Eqs. (1) and (2), the result of gSS(ADP)/gSS(KDP) can be obtained to be 1.58.
| Table 1. Raman spectroscopic parameters of ADP and KDP crystals. |
At room temperature, both ADP and KDP crystals have the same point group D2d, which belongs to space group 

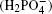
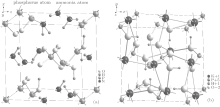
 | Fig. 5. Bond structures of ADP (a) and KDP (b) crystals, [15] All of the chemical bonds are represented by different kinds of lines labeled with bond length (Å ). |
adjacent anions 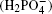
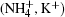
In this paper, the Raman gain coefficients of ADP and KDP crystals are compared with each other. The results show that although in the visible waveband gss(ADP) is obviously larger than gss(KDP), for the ultra-violet THG wavelength of Nd:YAG laser (355 nm) gss(ADP) is almost the same as gss(KDP). From the variation trend we can speculate that at the FHG wavelength 266-nm gss(ADP) is very likely to be smaller than gss(KDP), which needs more experiments to be confirmed. The present research results show that in the UV light region the SRS effect of ADP is comparable to or even weaker than that of KDP. The present research results also show that, as a powerful competitor to KDP crystal, ADP crystals could be used in large aperture THG or FHG processes.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|