Project supported by the State Key Development Program for Basic Research of China (Grant No. 2012CB932302) and the National Natural Science Foundation of China (Grant Nos. 10974235 and 11174336).
In situ high-pressure angle dispersive x-ray diffraction experiments using synchrotron radiation on Te nanoplates were carried out with a diamond anvil cell at room temperature. The results show that Te-I with a trigonal structure transforms to triclinic Te-II at about 4.9 GPa, Te-II transforms to monoclinic Te-III at about 8.0 GPa, Te-III turns to rhombohedral Te-IV at about 23.8 GPa, and Te-IV changes to body centered cubic Te-V at 27.6 GPa. The bulk moduli B0 of Te nanoplates are higher than those of Te bulk materials.
The high-pressure (HP) behavior of the group VI element tellurium (Te) has been investigated for a long time.[1– 6] At ambient conditions, Te is a semiconductor. It has a trigonal (Te-I) crystal structure with highly anisotropic characteristics consisting of helical chains along the c axis. The three atoms in the unit cell are linked by covalent-like bonds to the nearest neighbors along the chain and via van der Waals interactions to the second neighbors lying along adjacent chains.[7] Te has a series of complicated phase transitions under high pressure. It should be noted that the structural behavior within the pressure range of 4– 29 GPa has remained controversial so far.[1– 6] In the past, a generally accepted phase transition sequence was that trigonal Te-I changes to monoclinic Te-II at about 4 GPa, monoclinic Te-II turns to orthorhombic Te-III at about 7 GPa, orthorhombic Te-III transforms to rhombohedral Te-IV at about 11 GPa, and rhombohedral Te-IV changes to body centered cubic (bcc) Te-V at about 27 GPa.[1– 3] However, these results have been suspected by a diffraction study of Te-III and Te-IV.[4] In Ref. [4], it was suggested that Te-III exists in the pressure range of 7– 27 GPa and the structure of Te-III is monoclinic, which is different from the previous result.[2] Later, x-ray diffraction (XRD) showed a rather different HP scenario as follows. Trigonal Te-I partially transforms to triclinic Te-II at 4 GPa, and Te-I and Te-II coexist from 4 GPa to 4.5 GPa. Then, incommensurately modulated (IM) monoclinic Te-III appears at 4.5 GPa. Te-II and Te-III coexist from 4.5 GPa to 8 GPa, and Te-II disappears at 8 GPa while Te-III exists until 29 GPa. Finally, Te-III transforms into the bcc Te-V above 29 GPa.[5, 6]
It should also be noted that studies on the phase transition behaviors of Te under pressure have only been focused on Te bulk or power so far. During the past few decades, semiconductor nanomaterials (such as wires, rods, belts, tubes, spheres, plates, and flowers) have attracted significant interest for their excellent properties on future electronic, photonic and life-science applications. As a typical semiconductor, Te-I has a narrow direct band gap (band gap energy 0.35 eV) at ambient conditions. It exhibits many unique properties such as photoconductivity, catalytic activity, piezoelectricity, thermoelectricity, and nonlinear optical responses, [8– 11] which make it a multifunctional material in many electric and optoelectronic devices, such as self-developing holographic recording devices, radiative cooling devices, gas sensors, field-effect devices, and infrared acousto-optic deflectors.[10, 12, 13] Besides, the controlled growth of multi-morphology Te crystals (such as one-dimensional nanorods and nanowires, and two-dimensional hierarchical flowerlike microarchitecture)[11, 14– 19] has developed maturely, which makes it possible to be used in nanodevices. Generally, the nanomaterials have many promising physical properties in strength, thermal, electronic, and magnetic aspects in contrast with the conventional bulk materials. Especially, their surface features and energy band structures exhibit remarkable differences. So the structural transition of nanomaterials under pressure has become a hot point. However, the transition behaviors of Te nanomaterials under pressure have rarely been investigated so far.[20] A study focuses on the relationship between the phase transition pressure and grain size. It is found that the phase transition pressures are higher for the smaller nanocrystals (NCs).[20] In Ref. [20], the Te NCs were compressed up to 20 GPa which is low to observe the whole process of the phase transition. Considering the importance of determining the structural transition behaviors of Te nanomaterials under pressure, the structures were reexamined until about 29.6 GPa in this paper. We report the structural stability of Te nanoplate using the in situ high-pressure angle dispersive x-ray diffraction (ADXD) technique.
The Te nanoplates are the by-products of the CdTe nanocrystals. The thiol-capped CdTe nanocrystals were obtained using a chemical approach with hydrazine hydrate in an aqueous solution of Cd(NO3)2, mercaptoethylamine and tellurium. In an alkaline aqueous solution, hydrazine hydrate reduced commercial Te to be an active reactant, which was further reduced to negative bivalent telluride for the preparation of CdTe nanocrystals. If Ethylenediamine Tetramethylenephosphonic acid is added into the solution at the boiling point (the pH value decreases in solution) and the solution is kept at the temperature for 2 h, CdTe nanocrystals decompose and suspension occurs. After centrifuge, the Te nanoplates are obtained. The thickness of the nanoplates is tens of nanometers and the width is 500– 800 nm, which depend on the reaction time.
The ADXD experiment on Te nanoplates under external pressure from 0 to about 29.6 GPa at room temperature was performed at the Beijing Synchrotron Radiation Facility (BSRF). The x-ray wavelength was 0.6199 Å and the beam size was 35 × 15 μ m2 in diameter. High pressure was generated by using a symmetric type DAC with 300 μ m culets. The sample was loaded into the sample chamber in a T301 stainless steel gasket, and the pressure was calibrated by using the ruby luminescence technique. The diffracted x-rays were recorded with a MAR345 image plate detector placed at a distance of about 349.35 mm, which was calibrated by CeO2. The FIT2D program was used to display and integrate the diffraction rings on the image plate. No pressure transmitting medium was used in our experiments.
At ambient conditions, Te bulk materials crystallize in a trigonal structure with a space group of P3121. For Te nanoplates, they possess the same structure as the Te bulk materials at ambient conditions. Figure 1 shows the high-pressure in situ ADXD patterns of Te nanoplates up to about 29.6 GPa. With increasing pressure, all the diffraction peaks shift to a higher scattering angle, which indicates a gradual reduction of the unit cell volume. There are four phase transitions during loading pressure (Figs. 1(a)– 1(c)). The first phase transition of Te nanoplates occurs at about 4.9 GPa, indicated by the appearance of diffraction peaks positioned at about 12° , 12.3° , 12.6° , 14.3° , and 14.6° (marked by the upward arrows) and by the decrease of the original peaks (marked by the downward arrows) in Fig. 1(c). The first phase transition pressure (4.9 GPa) of the Te nanoplates is similar to that of Te powder (4.5 GPa).[5] Above 4.9 GPa, trigonal Te-I changes to Te-II, nevertheless the structure of Te-II has still been disputed so far. In the past, it was reported as a monoclinic structure with 4 atoms per unit cell and a structure comprising puckered layers through alternating long and short bonds, which contains zig-zag chains.[1] Recently, the structure of Te-II was reexamined by Hejny et al.[5] It was found that Te-II has a triclinic structure, whose superspace group is 

The second phase transition of Te nanoplates starts from at about 8.0 GPa, characterized by the decrease of the peak positioned at about 15° (Fig. 1(c)). On further compression, the three diffraction peaks at about 12° and the two diffraction peaks at about 15° are merged, which are all marked by the downward arrows as shown in Fig. 1(b). It should be noted that the structure of Te-III is still controversial up to now. It was previously accepted as an orthorhombic phase, [2] but this conclusion was disturbed by a subsequent diffraction study of Te-III and Te-IV up to 33 GPa, [4] which suggested that Te-III is a monoclinic structure. After that, the high pressure experiment of Te suggested that Te-III has an incommensurate monoclinic structure with superspace group I′ 2/m(0q0)s0.[5] According to the relevant report, [5] many of the Te-II reflection peaks could be interpreted as the splitting of Te-III reflection peaks. This interpretation is coincident with our results. The diffraction peaks at 15.1 GPa in our experiment can be assigned by the structure of monoclinic Te-III, whose superspace group is I′ 2/m. It was reported that the phase transition pressures in Te nanocrystals (NCs) goes higher for smaller ones. The two phase transitions pressures are 7.2 GPa and 10.3 GPa, 5.9 GPa and 8.8 GPa, and 4.0 GPa and 6.8 GPa, [20] respectively, for 20 nm Te NCs, 90 nm Te NCs, and bulk Te. The Te nanoplates used in our ADXD experiment is tens of nanometers thick and 500– 800 nm in width, which is larger than the NCs used in Ref. [20], and show phase transitions at 4.9 GPa and 8.0 GPa which are lower compared to the NCs but higher than that of bulk Te, consistent with the results of Ref. [20].
The phase transition from Te-III to Te-IV was characterized by the disappearance of some original diffraction peaks (marked by the downward arrows) and the appearance of new peaks (marked by the upward arrows) at 23.8 GPa as shown in Fig. 1(b). According to the relevant studies, [2, 3] these new peaks can be assigned to (001), 

The fourth phase transition of Te nanoplates happens at about 27.6 GPa, indicated by the appearance of diffraction peaks positioned at about 14.5° (marked by the upward arrows in Fig. 1(a)). This means that the Te nanoplates transform from a rhombohedral structure to a bcc structure (Te-V).
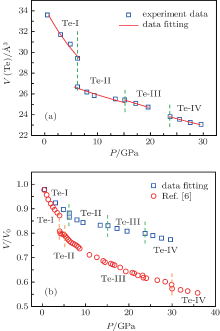 | Fig. 2. (a) The volume change of Te nanoplates and (b) the compressibility of Te nanoplates up to 29.6 GPa. |
The volume of one Te atom under different pressures can be well derived from the refined lattice parameters and the pressure– temperature– volume dependence can be decided systematically by an appropriate equation of state. The Birch– Murnaghan (BM) equation[21, 22] of the third order is valid for the isotropic case in a quasi-hydrostatic pressure environment. It is described as
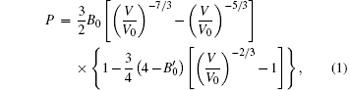
where V and V0 represent the volumes of one Te atom at pressure P and ambient pressure, respectively, while B0 and 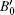
| Table 1. The bulk modulus B0 and zero-pressure volume V0 obtained from the BM equation. |
The relative volume change V/V0 of Te nanoplates as a function of pressure is shown in Fig. 2(b). The volume change behavior of Te powder under pressure cited from Ref. [6] is also plotted in Fig. 2(b) for comparison. The volume change of Te nanoplates is smaller than that of Te powder under the same pressure, as shown in Fig. 2(b), which indicates that the Te nanoplates are more difficult to compress due to the size effect than Te powder under the same compression conditions. It should be noted that the B0 is getting smaller for Te-III and Te-IV. At the moment, we do not know the reason for this, but a similar phenomenon was also reported by other groups in Ho2O3 and Dy2O3 compounds.[25, 26]
An in situ high-pressure ADXD experiment for Te nanoplates was performed at room temperature. The results show that Te-I with a trigonal structure transforms to triclinic Te-II at ∼ 4.9 GPa, Te-II transforms to monoclinic Te-III at ∼ 8.0 GPa, Te-III turns to rhombohedral Te-IV at ∼ 23.8 GPa, and Te-IV changes to bcc Te-V at 27.6 GPa. By fitting the relative volume versus pressure, it is shown that the bulk modulus B0 of Te nanoplates is higher than that of Te bulk materials.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|




