Corresponding author. E-mail: xin_wang@jlu.edu.cn
Project supported by the National Natural Science Foundation of China (Grant Nos. 51172194 and 51172091), the Program for New Century Excellent Talents in University, China (Grant No. NCET-12-0240), and Jilin Province Science and Technology Development Program, China (Grant No. 20130101023JC).
Double perovskite oxide Sr2CoFeO6 (SCFO) has been obtained using a high-pressure and high-temperature (HPHT) synthesis method. Valence states of Fe and Co and their distributions in SCFO were examined with X-ray photoelectron spectroscopy. The electric transport behavior of SCFO showed a semiconductor behavior that can be well described by Mott’s law for variable-range hopping conduction. The structural stability of SCFO was investigated at pressures up to 31 GPa with no pressure-induced phase transition found. Bulk modulus B0 was determined to be 163(2) GPa by fitting the pressure–volume data to the Birch–Murnaghan equation of state.
Double perovskite oxides of general formula A2BB′ O6 (A = divalent alkaline earth ions; B and B′ = transition metal ions) have been extensively studied because they present interesting magnetic and transport properties. Especially, the mixed-valent molybdenum perovskite (Sr2FeMoO6) exhibits colossal magnetoresistance, [1] and the copper oxide (Sr2YRu0.95Cu0.05O6) shows superconductivity.[2] In either case, the special physical properties are strongly related to the charge ordering phenomena and the couplings among lattice, orbital, and spin degrees of freedom.
Among those oxides, Co-based double perovskite oxides have attracted a lot of attention and shown a wide range of intriguing properties, e.g., antiferromagnetism, ferromagnetism, [3– 9] spin-glass behavior, [10] and magnetoresistance.[11] Sr2CoMoO6 is an insulating antiferromagnet with electronic configuration Co2+ (3d7)– Mo6+ (4d0). This configuration requires a completely unquenched orbital contribution for the high spin (HS) Co2+ cation, which explains the large effective moment of 5.3μ B obtained in the paramagnetic region well above TN.[11] By substituting Mo with Fe, the different valence states of Co and Fe may have interesting impacts on the magnetic and transport properties of the new oxide. To the best of our knowledge, only a few studies on Sr2CoFeO6 have been reported.[10, 12, 13] The previous study on Sr2CoFeO6 showed that Fe and Co were both in tetravalent oxidation states, and the HS states of 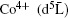
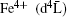
In recent studies, temperature- and pressure-induced structural changes have been observed in Co-based double perovskite oxides. For example, the structure of Sr2CoSbO6 would transform from trigonal 


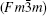
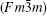

The precursor of Sr2CoFeO6 was prepared by the sol– gel method with citric acid as a complexant. Stoichiometric Sr(NO3)2, Co(NO3)2· 6H2O, and Fe(NO3)3· 9H2O of analytical grade were dissolved in water, and then citric acid and ethylene glycol were added. The mixed solution was slowly evaporated at 80– 90 ° C until a gel was formed. Subsequently, the gel was dried at 130 ° C and then heated at 850 ° C in air for 10 h. After being uniformly ground in an agate mortar, the mixture was pressed into a disk with a diameter of 8 mm and a thickness of 2 mm. The disk-shaped samples were assembled for the high-pressure and high-temperature (HPHT) synthesis in a cubic anvil high-pressure apparatus at 1473 K and 5 GPa for 1 h.
The crystal structure and the phase purity of Sr2CoFeO6 were examined using conventional X-ray powder diffraction at room temperature. X-ray diffraction (XRD) measurement was performed using a Rigaku Rotaflex X-ray diffractometer with Cu Kα radiation in the range of 20° – 80° . High-pressure angle dispersive XRD measurement was performed at beamline 3W1A of the Institute of High Energy Physics, CAS.
X-ray photoelectron spectroscopy (XPS) measurements were performed with an ESCALAB 250 spectrometer using an Al Kα X-ray source. The obtained binding energies were standardized and analyzed using C1s as the reference at 284.9 eV.
Measurements of the electric resistivity were performed with the four-point probe method from 10 K to 300 K under an applied magnetic field of 8 T in a commercial Quantum Design physical property measurement system (PPMS).
At ambient pressure, Sr2CoFeO6 is found to have a cubic crystal structure (space group Fm-3m). As shown in Fig. 1, it can be viewed as a regular arrangement of the corner-sharing CoO6 and FeO6 octahedra, alternating along the crystal axes, with the large Sr cations occupying the voids between CoO6 and FeO6 octahedra. The X-ray diffraction pattern at the ambient condition is displayed in Fig. 2, and the calculated lattice parameter a = 7.7323(5) Å is in good agreement with the previous reports.[12, 25]
Figure 3 shows the results of XPS measurements on Fe 2p and Co 2p in Sr2CoFeO6. Lorenz– Gaussian fitting of the peaks to the components of Fe3+ , Fe4+ , Co3+ , and Co4+ is also given in the figure. The relative peak intensities of the fitting curves are summarized in Table 1. The peaks at 712.51 eV and 725.63 eV identify the existence of Fe4+ ; those at 710.56 eV and 723.64 eV identify the existence of Fe3. The binding energies of 780.59 eV and 795.77 eV correspond to Co3+ ; while those of 781.66 eV and 797.55eV correspond to Co4+ . These results are in good agreement with the previous reported values.[26, 27]
| Table 1. Relative XPS peak intensities of Fe3+ , Fe4+ , Co3+ , and Co4+ components. |
According to the XPS spectral analysis, it can be deduced that in Sr2CoFeO6, the B sites are randomly occupied by Fe and Co in the mixed valence states of Fe3+ /Fe4+ or Co3+ /Co4+ . Owing to the coexistence of Fe4+ , Fe3+ , Co4+ , and Co3+ , in order to maintain electric neutrality of Sr2CoFeO6, there should be some oxygen vacancies inside Sr2CoFeO6. Such oxygen vacancies induced by the random occupancy and the mixed valence may account for the observed spin glass behavior, which is caused by the inhomogeneous magnetic exchange interactions.[10]
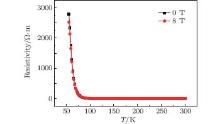
The electric resistance of SFCO in 0 T and 8 T applied magnetic fields is presented in Fig. 4. It exhibits a monotonic increase with decreasing temperature, and the variation of the electric resistance indicates a semiconducting behavior.
As shown in Fig. 4, unlike Sr2CoMoO6, the resistivity curves measured in the fields of 0 T and 8 T are almost identical, indicating that Sr2CoFeO6 has a negligible magnetoresistance.
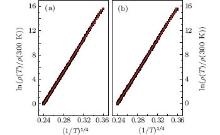
In order to understand the electric transport properties of Sr2CoFeO6, the resistivity data are fitted to various models, among which the Mott variable-range hopping (MVRH) model[28– 30] is the best fit to our results, as shown in Fig. 5. The Mott variable-range hopping model gives the temperature-dependent resistivity as follows: ρ = ρ oexp(To/T)x, with exponent x = 1/4, as expected in three dimensions. The best fitted values are To = 3.10 × 108 K, ρ o = 1.34 × 10– 14 Ω · m (at 0 T) and To = 3.06 × 108 K, ρ o = 1.51 × 10− 14 Ω · m (at 8 T), where To is related to the localization length ξ with the relationship 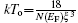
Various compounds in the double perovskite family are found to have different high pressure behaviors.[23, 24] Further investigations of the structural stability in these compounds would be beneficial for understanding their physical properties at high pressures. This study reports a high pressure X-ray diffraction study of Sr2CoFeO6. Figure 6 shows the X-ray diffraction patterns at various pressures up to 31.0 GPa. The lowest diffraction pattern is indexed with respect to the cubic phase and some impurity (unreacted oxides) peaks existing in the pattern are marked by asterisks. All the X-ray diffraction peaks shift toward higher 2θ values, and no new peaks are found with increasing pressure. The variation of the relative intensities and the broadening of the X-ray diffraction peaks upon compression can be attributed to the thinning of the sample and the uniaxial compression in the process. We thus conclude that no structural phase transition has occurred up to the highest pressure.
The lattice parameter a and the unit cell volume as a function of pressure are plotted in Fig. 7. The second order Birch– Murnaghan equation of state (BM-EoS) is applied to determine the bulk modulus.[31] The fitting gives the bulk modulus Bo = 163(2) GPa. The lattice parameter and the unit cell volume of Sr2CoFeO6 decrease smoothly with increasing pressure, with no evidence of any phase transition throughout the whole pressure range.
Double perovskite Sr2CoFeO6 has been successfully prepared by the HPHT method. Using X-ray photoelectron spectroscopy, we confirm the existence of different valence states of Fe and Co ions, and their random distributions in the lattice of Sr2CoFeO6 are also shown. The resistivity measurements show that Sr2CoFeO6 is a Mott variable-range hopping semiconductor with a large To of 3.06 × 108 K and it exhibits a negligible magnetoresistance in a magnetic field of 8 T. In situ synchrotron X-ray diffractions of Sr2CoFeO6 have been performed at pressures up to 31.0 GPa in a diamond anvil cell. The crystal structure of this compound remains cubic in the whole pressure range. The bulk modulus of Sr2CoFeO6 is determined to be 163(2) GPa.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|