†Corresponding author. E-mail: wcz@jlu.edu.cn
*Project supported by the National Key Basic Research Program of China (Grant No. 2015CB251103), the Development Program of Science and Technology of Jilin Province, China (Grant No. 20140101093JC), and the Program of Science and Technology of Jilin City, China (Grant No. 201434006).
Spinel LiCoMnO4 is prepared by solid-state reaction and its magnetic properties are comprehensively studied by direct current (DC) and alternating current (AC) susceptibilities, isothermal remanent magnetizations, and magnetic hysteresis. Fitting to the Curie–Weiss law by using high-temperature zero-field-cooled susceptibility confirms a low-spin state of Co3+ with S = 0. Both the fitting parameters first increase and then tend to be saturated at high magnetic fields through using isothermal remanent magnetizations, which suggests a spin glass transition at low temperature. AC susceptibility study also supports this conclusion since the frequency dependence of peak position and intensity follows the tendency of a spin glass transition. The origin of the spin-glass transition in LiCoMnO4 might be attributed to a spatial segregation between non-magnetic Co3+ regions and spin glass ordered regions of Mn4+ ions.
With the growing demand for the high-performance lithium ion batteries (LIBs), LiMn2O4 with spinel structure has attracted much attention owing to the low cost of manganese and one-dimensional Li-ion migration pathway.[1– 4] LiMn2O4 crystallizes in the space group Fd-3m, and Li, Mn, and O occupy 8a, 16d, and 32e sites, respectively. Owing to the redox Mn3+ /Mn4+ , LiMn2O4 shows a reversible capacity of nearly 135 mA· h· g− 1.[4] However, the unsatisfactory cycle stability and voltage degradation prevent it from being further used as the potential cathode material for LIBs. Many efforts have been made to improve its electrochemical performance via partially substituting Mn by Co, Ni, Cr or Fe.[5– 8]
A study on the crystallographic, electronic and magnetic structure of the cathode material is meaningful for the understanding of the electrochemical property since cation ordering and phase stability are closely related to the charge capacity and capacity retention. LiMn2O4 has been found to show a magnetic transition from paramagnetism to spin glass (SG) because of the geometrical frustration.[9] When Mn ions are partly substituted by Cr, an SG is also found in LiCrMnO4 due to the random distribution of Cr3+ and Mn4+ ions.[10] LiCoMnO4 is another attractive cathode material because it can provide a high voltage plateau above 5 V.[5] Electron paramagnetic resonance (EPR) spectrum of LiCoMnO4 suggests a low-spin state of Co3+ state with S = 0.[11] Interestingly, if Co3+ ions occupy 16d site homogeneously, the nonmagnetic ions will act as the dilution to the magnetic interaction and paramagnetism is expected. In this paper, an SG transition is proposed in LiCoMnO4 by the comprehensive magnetic method. In contrast to the expected paramagnetism, the existence of SG transition can be explained on the basis of cation random distribution in LiCoMnO4.
LiCoMnO4 was prepared by a solid-state reaction. Stoichiometric mixtures of Li2CO3, Co2O3, and Mn2O3 were ground thoroughly, and fired at 650 ° C or 6 h and then at 850 ° C or 24 h before cooling to room temperature naturally. The crystal structure of the as-prepared sample was studied by x-ray diffraction (XRD) in capillary transmission mode on a STOE STADI P diffractometer with Mo Kα 1 radiation. Diffraction data were recorded over a 2θ range of 5° – 80° in steps of 0.01° . The software Fullprof was used for data analysis and Rietveld refinement of the structural model. Magnetic characterization was performed by a superconducting quantum interference device magnetometer (Quantum Design MPMS-XL).
Figure 1 shows the XRD pattern for the as-prepared LiCoMnO4. Rietveld refinement confirms the spinel structure with space group of Fd-3m and no impurity phase can be detected. Detailed refinement parameters are listed in Table 1. Note that the relative intensities of (311) and (400) peaks are different from those of LiMn2O4, [12] which may come from the Td and 16c defects due to the random distributions of Mn4+ and Co3+ ions at the 8a and 16c sites, respectively. The degrees of Td and 16c defects are calculated to be 14.9% and 0.9%, respectively, by means of the Rietveld refinement.
 | Fig. 1. (a) Observed and calculated x-ray diffraction profiles together with their difference for the as-prepared LiCoMnO4 compound; (b) Schematic diagram for the crystal structure of spinel LiMn2O4. |
| Table 1. LiCoMnO4 crystal structure parameters determined by Rietveld refinement. |
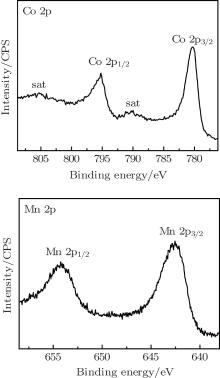
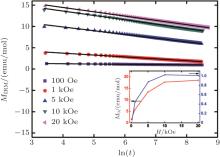
XPS spectra were carried out to further study the valence states of Co and Mn ions as shown in Fig. 2. In the Co 2p spectrum, the binding energies of the peaks located at 780.2 eV and 795.2 eV correspond to 2p3/2 and 2p1/2 of Co3+ , in accordance with those reported in LiCoO2, along with two satellite peaks at 790.1 eV and 805.3 eV.[13] In the Mn 2p spectrum, the peak located at 642.5 eV agrees with the 2p3/2 of Mn4+ as reported in Ref. [14].
Zero-field cooled (ZFC) and field-cooled (FC) susceptibilities from 5 K to 300 K under an applied field of 1000 Oe (1 Oe= 79.5775 A· m− 1) are exhibited in Fig. 3 with the inverse susceptibility curve as indicated in the inset. At high-T, the susceptibility increases with the decrease of temperature, indicating a paramagnetic behavior. By fitting the Curie-Weiss law using high-temperature paramagnetic data, Curie and Weiss constants are calculated to be C = 1.84 emu· K/mol· Oe and θ = − 49(1) K, respectively. The negative Weiss constant indicates the antiferromagnetic interaction between spins in LiCoMnO4. By employing the equation 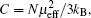


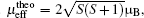
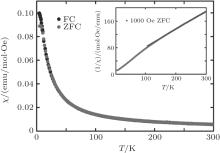 | Fig. 3. ZFC/FC curves of LiCoMnO4 under 1000 Oe and reciprocal ZFC curve with the Curie– Weiss fitting in inset. |
At low-T, ZFC and FC curves begin to show an irreversible behavior at nearly 17 K, and ZFC branch displays a peak at 7 K, which is defined as the freezing temperature (Tf), reminiscence of an SG transition. To confirm the SG transition, the isothermal remanent magnetizations (MIRM) are recorded and shown in Fig. 4. Applying a procedure in which the sample is zero-field cooled to 5 K and different magnetic fields are applied for 10 min, a slow decay of the MIRM after the field is reduced to zero, which is a typical feature for the spin glass behavior below Tf.[16] By fitting the time dependence of magnetization using the formula MIRM(t) = M0 − α ln(t), we can obtain the fitting parameters M0 and α as a function of magnetic field (the inset of Fig. 4). Both the parameters first increase and then tend to saturate at high magnetic fields, which is a typical feature for SG system.[17]
The dynamic behavior of SG near Tf is also studied, and AC susceptibilities under different frequencies ranging from 1 Hz to 500 Hz are shown in Fig. 5. The height of the peak decreases and the peak position shifts toward the high temperature with the increase of frequency, in accordance with the characteristics of SG behavior. Supposing the existence of an equilibrium phase transition with a divergence of relaxation time (τ ) near the transition temperature, we employ the power law to explain the relaxation behavior in the SG system. The power law can be described as τ = τ 0(Tf/Tg − 1)− zv, where Tg corresponds to the relaxation temperature, τ 0 is related to the relaxation time of the individual magnetic moment, and zv is the dynamic critical exponent.[18] The values are calculated to be τ 0 = 6.2 × 10− 11 s and zv = 6.9, which fall in the typical values (zv ∼ 7.9 ± 1 K) determined by the simulations of Ogielski for a three-dimensional SG with short-range magnetic interactions.[19] This also confirms the SG transitions in spinel LiCoMnO4.
 | Fig. 5. Temperature dependences of the real-part of AC susceptibility in the frequency range from 1 Hz to 500 Hz, and the fitting to power law (in inset). |
The field dependences of magnetizations (Fig. 6(a)) under different temperatures are recorded, with the magnified curves indicated in the inset. The magnetization shows a linear dependence on magnetic field at 50 K. The small magnetic hysteresis appears as temperature decreases below Tirr. The coercive field and remanence at 5 K are − 201 Oe and 3.4 × 10− 3 μ B/Mn, respectively. Note that there is no magnetic saturation at 5 K even under a strong field (5 T), which is consistent with the competition nature between antiferromagnetic and ferromagnetic interaction for SG. The Arrott plot (Fig. 6(b)), obtained from magnetization isotherms, shows strong curvature toward the H/M axis without any intercept on the M2 axis, indicating no sign of the spontaneous magnetization.[20]
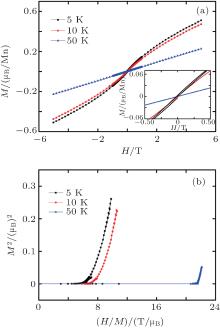 | Fig. 6. (a) Variations of magnetic field and magnification of specified parts (in inset) with magnetization and (b) Arrott plots (H/M versus M2), at different fixed temperatures of LiCoMnO4. |
Note that the geometrical frustration and disorder are two major reasons for the formation of SG. In the spinel LiMn2O4, the tetrahedral arrangement of Mn ions with the same bond distance would give a strong frustration effect and frustration parameter (f) equals 11. Correspondingly, an SG transition is observed in LiMn2O4 with Tf = 25 K.[9] When Mn ions are partly substituted by Cr, an SG is also suggested, but fCr is lower than 10, [10] which is considered as the critical value at which the geometrical frustration plays an effect. This means that the frustrated tetrahedral arrangement of Mn ions would be destroyed when Cr3+ ions are introduced into the lattice. The random distributions of Cr3+ and Mn4+ ions are likely to be responsible for the formation of SG. Usually, nonmagnetic ions act as the dilution to the magnetic interaction, and the doped compounds show paramagnetism. In LiCoMnO4, if the random distributions of Co3+ and Mn4+ ions are homogeneous, nonmagnetic Co3+ ions would destroy the exchange interaction between Mn4+ ions and paramagnetism is expected in the whole measured T region. But, interestingly, LiCoMnO4 shows an SG transition like LiCrMnO4, instead of the expected paramagnetism, and the frustration parameter equals 7, which also implies that nonmagnetic Co3+ ions destroy the frustrated lattice. The random distributions of Co3+ and Mn4+ ions are likely to result in the formation of different ions clusters due to the different electronic structures between Mn4+ ions 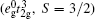

We study the structural and magnetic properties of LiCoMnO4 by Rietveld analysis and DC and AC susceptibilities. The fitting to the high-temperature Curie– Weiss law suggests the low spin state of Co3+ ions. The spin glass transition at low temperature is confirmed by the irreversibility between ZFC and FC curve, the isothermal remanent magnetizations, and the fitting to the AC susceptibility peak by using the power law. The origin of SG is attributed to spatial segregation between non-magnetic Co3+ clusters and spin glass ordered regions of Mn4+ clusters rather than geometrical frustration.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|