†Corresponding author. E-mail: gaolinyan@whu.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 51172168 and 51072139) and the Science Funds from the Ministry of Science and Technology, China (Grant Nos. 2014DFB50130 and 2011CB612304).
The effects of oxidation of DyH3 with respect to dysprosium addition to Nd–Fe–B sintered magnets are examined. Samples sintered with the addition of freshly milled dysprosium hydride, dysprosium hydride exposed to air at room temperature for 15 min and dysprosium hydride exposed to air at 100 °C for 3.5 hours are studied from the aspects of magnetic properties, microstructures, and their degradation, respectively. It is found that some oxidized dysprosium is distributed in the Nd-rich phase; hence, the decrease of remanence occurred. The degradation results indicate that pre-oxidised dysprosium can be a major factor in increasing the corrosion rate. The microstructures and corrosion acceleration test suggested that the oxidation is detrimental to remanence.
Nd– Fe– B magnets are widely applied in industrial manufacture, such as, voice core motor in hard disc drive, motors, generators, mobile phone, etc, due to their high magnetic performance.[1, 2] It is important for devices converting electrical to mechanical energy to have strong magnets with minimized volume.[3] In order to improve the magnetic performance of Nd– Fe– B magnets, many additive elements have been studied.[4, 5] The use of alloy powders as additives to blend with the Nd– Fe– B sintered magnets by grain boundary reconstruction is another way to achieve better magnetic properties.[6, 7] In particular, dysprosium addition was considered as an important method because Dy2Fe14B compounds have a higher anisotropy field than Nd2Fe14B.[8, 9] Previous work proposed a model that Dy-rich phases react with the grain boundary of matrix phases, substituting Nd to form Dy2Fe14B grain surface layer, while the inner core of Nd2Fe14B phases is Dy-lean.[10]
Dysprosium metal is more difficult to be obtained than Dy2O3. Consequently, Dy2O3 addition is selected[11] and DyHx[12, 13] as Dy sources is chosen for comparison. With Dy2O3 or DyHx addition, the magnetic properties of Nd– Fe– B magnets were examined. Since some oxidized dysprosium which is distributed in the Nd-rich phase may play a role in pinning Dy into the matrix phase, and the evolution of dysprosium hydride to dysprosium oxide will occur when DyHx is blended-in Nd– Fe– B magnets, we investigate the oxidation of DyH3 in Nd– Fe– B sintered magnets with dysprosium addition. Besides, the poor corrosion resistance of Nd– Fe– B magnets limits their life span. Corrosion occurs initially along the grain boundaries, hydrogenation and oxidation also contribute to its degradation.[14] Hence, it is important to observe the effect of oxidation of DyH3 in the degradation of Nd– Fe– B magnets.[15] In this paper, an effort to control the dysprosium distribution in the Nd-rich phase and grain boundaries is attempted by blending oxidized dysprosium hydride before sintering.[16] The present investigation of degradation of these addition-blended Nd– Fe– B sintered magnets, examined using an environmental chamber, agrees well with the previous studies.[17, 18] The results of these experiments are presented.
Dysprosium metal was hydrided at 380 ° C– 420 ° C for 30 mins with a hydrogen pressure of 8 bars (1 bar = 105 Pa) to completely convert it to dysprosium hydride. It was then milled in cyclohexane for 20 hours giving fine hydride particles (3 μ m– 7 μ m). Sample I was made by blending Nd16Fe76B8 magnets with freshly milled 3 at.% DyH3. Samples II and III were made similarly but the DyH3 was exposed to air for 15 minutes at room temperature and for 3.5 hours at 100° C, respectively. These blended powders were sealed in a rubber tube, aligned in a 4.5-T magnetic field and isostatically pressed (1200 kg· cm– 2). The 60% dense green pacts were sintered in a vacuum furnace at 1020 ° C for 1 hour and then cooled in the furnace.
The sintered magnets were sliced and polished to 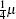
As DyH3 was exposed to air, it is changed from dark brown to light yellow giving out heat, this is presumably due to the oxidation reaction Eq. (1) occurring on the surface of DyH3 particles:
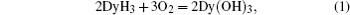 |
 |
The oxidised DyH3 was blended in Nd16Fe76B8 hydrides for sintering when the reaction Eq. (2) occurred and Dy2O3 was introduced in Nd16Fe76B8 magnets. The magnetic properties of samples I, II, and III were measured by permeameter, the degmagnetisation curves are shown in Fig. 1. The values of coercivity, remanence, and densities are illustrated in Fig. 2.
 | Fig. 1. Demagnetisation curves of Nd16Fe76B8 + 3-at.% DyH3 sintered at 1020 ° C for 1 hour and then cooled in the furnace for samples I, II, and III. |
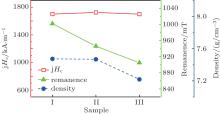 | Fig. 2. Coercivity, remanence and density of Nd16Fe76B8 + 3-at.% DyH3 sintered at 1020 ° C for 1 hour and then cooled in the furnace for samples I, II, and III. |
From Fig. 1 and Fig. 2, it can be seen that the coercivities of samples I, II, and III are almost the same. There is not too much effect of oxidation of DyH3, at least the oxidation does not damage the coercivity. However, remanences decrease due to the oxidation. This happens because the blending with oxidised DyH3 lowers wetability during the sintering and decreases the density of the sintered magnets and, hence, finally dilutes Nd2Fe14B(Φ ), the hard magnetic phase, per volume unit. On the other hand, the volume fraction of Nd2Fe14B matrix phase is reduced because of the introduction of Dy2O3 during sintering. It is easy to understand that in sample III the oxidation of the surface of DyH3 particles proceeds more than that of in sample II. Therefore, the decrease of both density and remanence is greater when DyH3 is oxidised more severely, see Fig. 2.
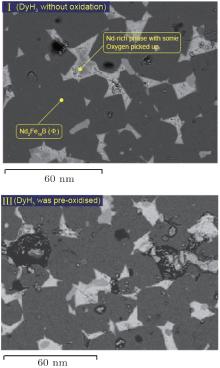
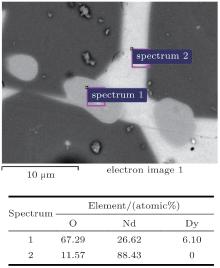
The microstructure and composition of magnets of Nd16Fe76B8 with the addition of 3-at.% DyH3, one freshly milled and the other oxidised at 100 ° C for 3.5 hours in air, sintered at 1020 ° C for 1 hour and then cooled in the furnace, typical samples I and III respectively, are shown in Fig. 3. This reveals a relative complete sintering structure in sample I. However, the Nd16Fe76B8 blended with pre-oxidised DyH3 has some porosity; e.g., in the microstructure of sample III. Although this is a shortcoming, a large amount of dysprosium is distributed as Nd– Dy– O phase in the Nd-rich phases, as is illustrated in Fig. 4. This means the pre-oxidised DyH3 can slow down the diffusion of Dy into the matrix phase, and can be distributed in Nd-rich phases. It is believed that Nd– Dy– O phase acts as a pinning site in the magnet. This could be a benefit to restricting the decrease of remanence due to diffusion of Dy in to the matrix phase.
3.3.1.Weight change rate of magnets during corrosion
The sintered magnets of samples I, II, and III were corroded in an environmental chamber at 85 ° C, RH: 80%. The corroded samples were dried in a drying oven for 30 minutes to remove moisture and then the weight of each sample was recorded. The relative weight change (Wn– W1)/W1 versus time is plotted and shown in Fig. 5. In the weight change rate formula (Wn– W1)/W1, W1 represents the weight before corrosion and Wn represents the weight of the sample after corrosion for n days. From this figure, the slope of the relative weight change rate is related to the addition blended in. The higher the slope of the weight change rate, the faster the degradation of the magnets. The slope of sample I is the smallest and this means that its corrosion resistance is the best among these four magnets. It should be pointed out that the slope of the relative weight change rate of sample III increased rapidly after corrosion for 8 days. It is conjectured that the Dy2O3 introduced by the reactions Eq. (1) and Eq. (2) mentioned above also acts as an anode during the corrosion. The DyH3 of sample II was less pre-oxidised than sample III, thus its corrosion resistance is better than that of sample III, this is proven by the different values of the slope of the relative weight change rates, as shown in Fig. 5.
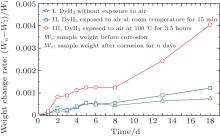 | Fig. 5. Weight change rates of the magnets (Wn – W1)/W1 versus time during the corrosion in the environmental chamber at 85 ° C, RH: 80%. |
3.3.2.Corrosion status after 18 days
The macroscopic corrosion status and microstructures of magnets of samples I, II, and III after corrosion at 85 ° C, RH@ 80% in the environmental chamber for 18 days are shown in Fig. 6. We can see clearly that sample III started to be powder and sample I is maintained well, only its surface is corroded and its colour has changed. The macroscopic corrosion status that we recorded agrees well with the weight change rates that we measured (as discussed above).
 | Fig. 6. The macroscopic corrosion status and microstructures of magnets of samples I, II, and III after corrosion at 85 ° C, RH: 80% in the environmental chamber for 18 days. |
It also can be seen in Fig. 6 that the degradation of sample III proceeds very quickly after corrosion at 85 ° C, RH:80% for 18 days, and the microstructure shows that the Nd-rich phase is severely corroded. Meanwhile, the relatively lower density of sample III, see Fig. 2, which is caused by the lower wetability due to the pre-oxidised DyH3 blended in, is the main reason for the sudden acceleration in degradation after 18 days corrosion. For sample I, although the corrosion started on the surface, the bulk magnet maintained well, see Fig. 6, this is due to a much lower penetration of corrosion, which is much slower than the rest of the samples.
The effects of oxidation of DyH3 in Nd– Fe– B sintered magnets with dysprosium addition have been examined. The magnetic property measurements show that remanence decreases as the oxides are introduced but coercivity is almost invariant. Microstructure examination reveals that certain amounts of dysprosium are distributed in the Nd-rich phase of the magnets with pre-oxidised DyH3 blended in, which may play a role in restricting Dy diffuse to the matrix phase. Degradation of the sintered magnets in an environmental chamber indicates that relative corrosion speeds are related with the oxides involved. From the point of view of application, the pre-oxidised of DyH3 as additions should be limited.
The authors would like to thank Philips Components Ltd. for the provision of dysprosium metal. The Department of Science and Technology, Jiangsu and Hubei Province and the Suzhou and Wuhan Science and Technology Bureau and other potential support is also acknowledged. The School of metallurgy and Materials, University of Birmingham, UK is also acknowledged for providing experimental facilities.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|