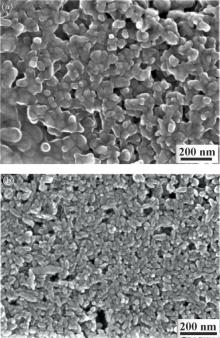
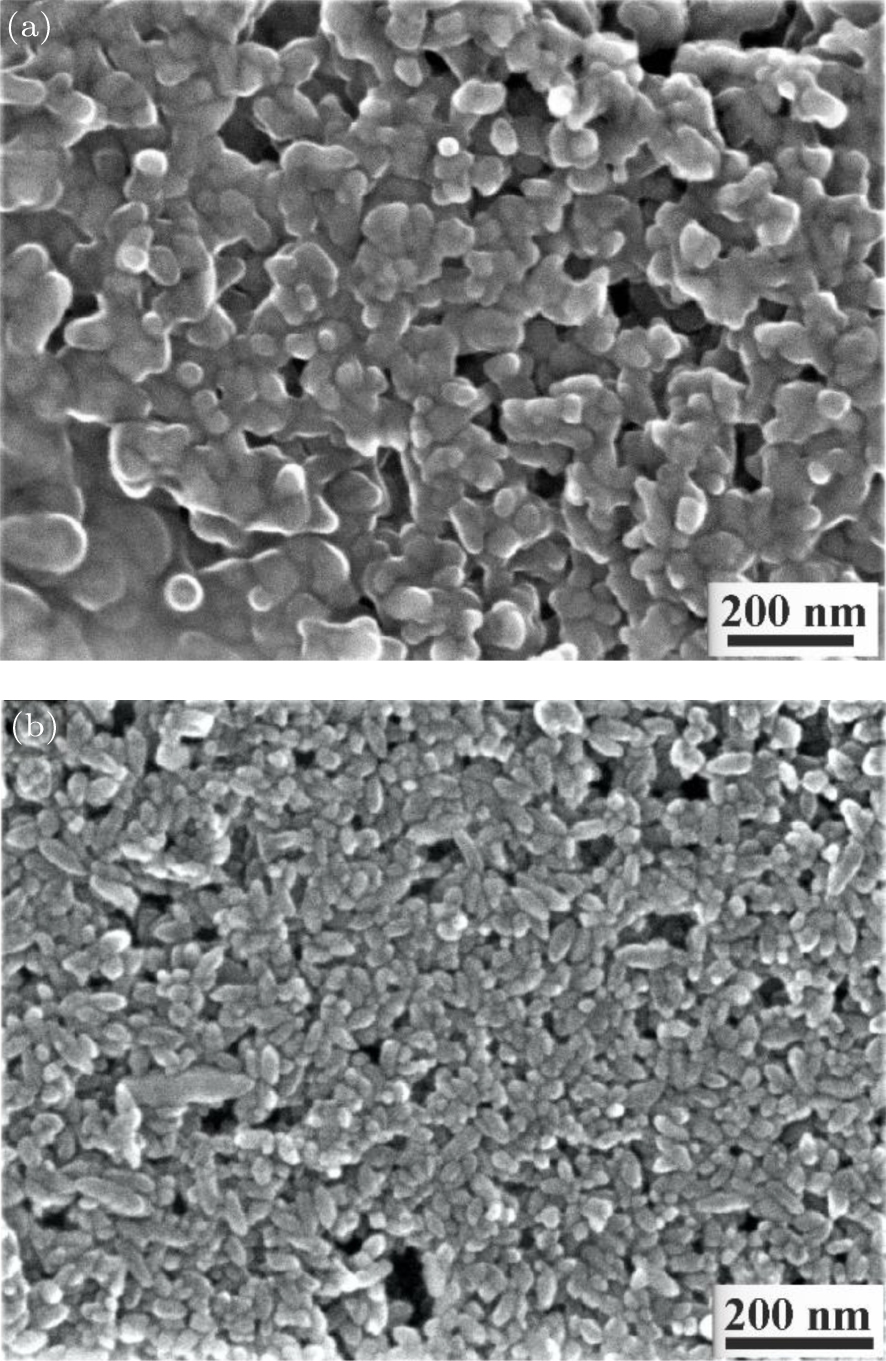
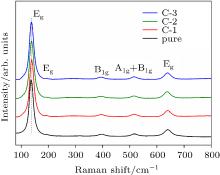
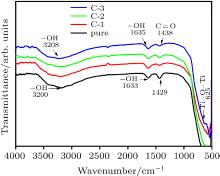
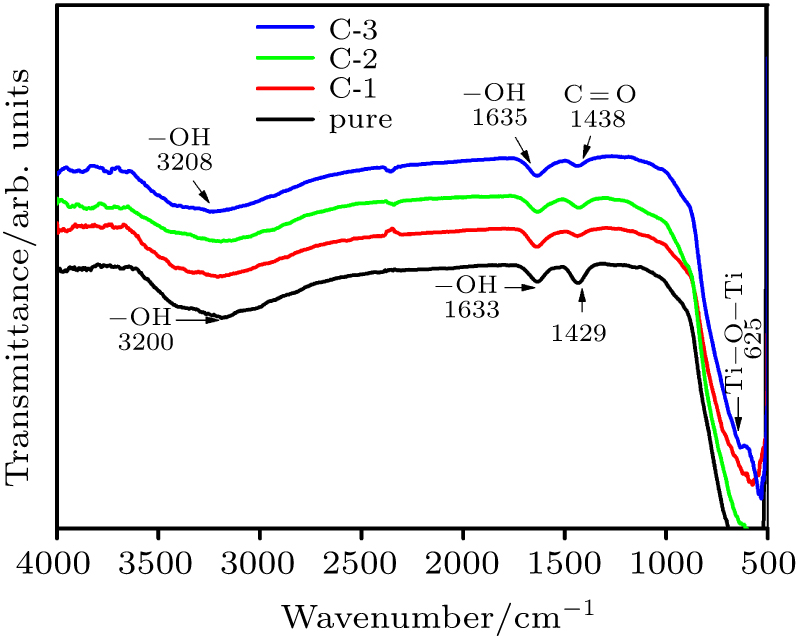
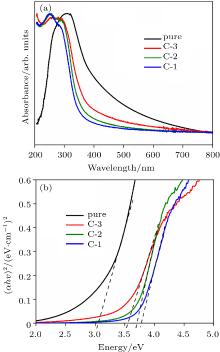
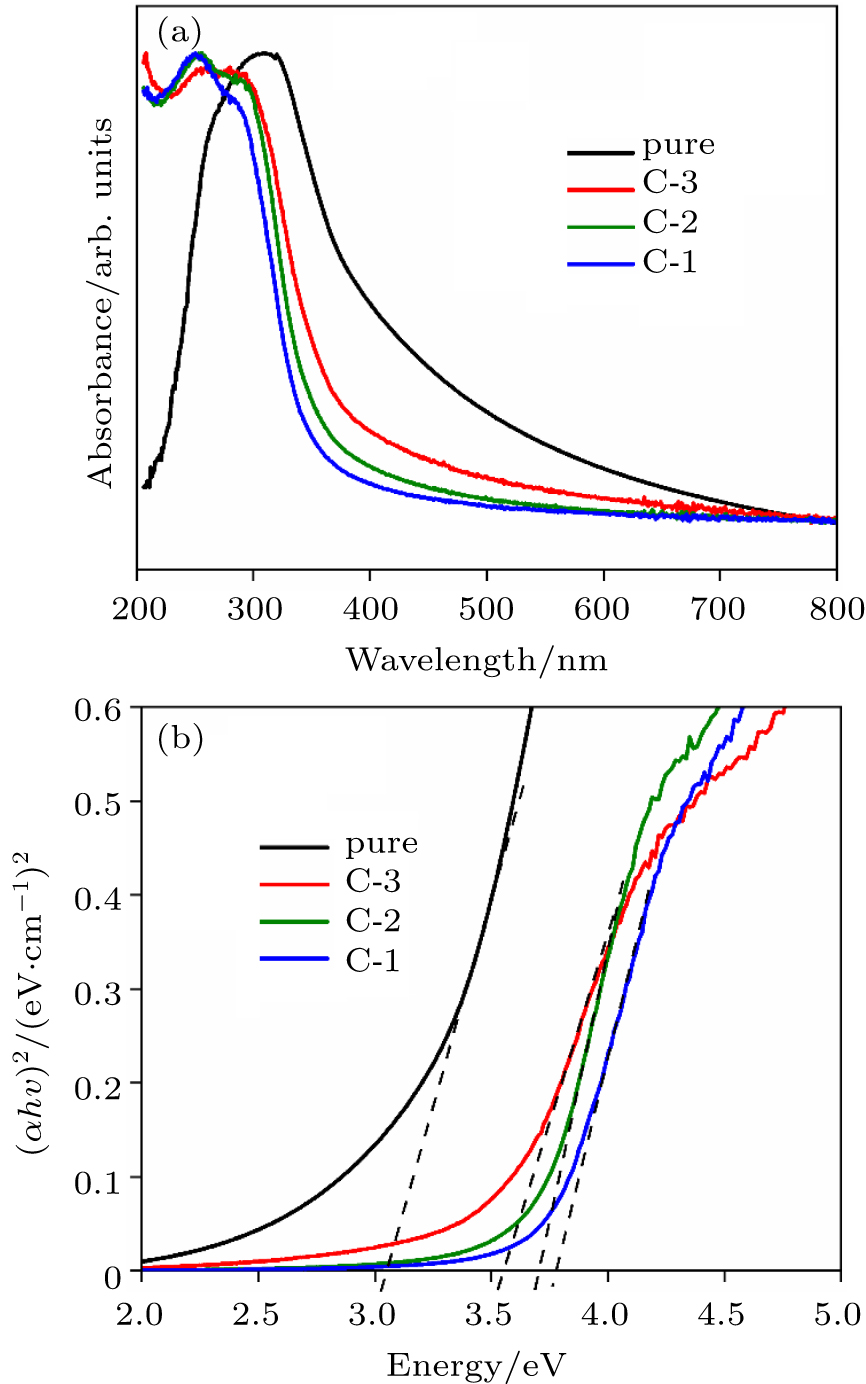
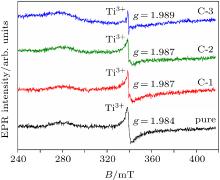
| [1] |
Khan A, Toufiq A M, Tariq F, Khan Y, Hussain R, Akhtar N, Rahman S U 2019 Mater. Res. Express. 6 065043 DOI: 10.1088/2053-1591/ab0aaf |
| [2] |
|
| [3] |
Zhang G, Xiao X, Li B, Gu P, Xue H, Pang H 2017 J. Mater. Chem. A 5 8155 DOI: 10.1039/C7TA02454A |
| [4] |
|
| [5] |
Vardi N, Anouchi E, Yamin T, Middey S, Kareev M, Chakhalian J, Dubi Y, Sharoni A 2017 Adv. Mater. 29 1605029 DOI: 10.1002/adma.201605029 |
| [6] |
|
| [7] |
Yin Z, Tordjman M, Lee Y, Vardi A, Kalish R, del Alamo J A 2018 Sci. Adv. 4 eaau0480 DOI: 10.1126/sciadv.aau0480 |
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
Huang Y, Guo Z, Liu H, Zhang S, Wang P, Lu J, Tong Y 2019 Adv. Funct. Mater. 29 1903490 DOI: 10.1002/adfm.v29.45 |
| [12] |
|
| [13] |
|
| [14] |
Smith S J, Stevens R, Liu S, Li G, Navrotsky A, Boerio-Goates J, Woodfield B F 2009 Am. Mineral. 94 236 DOI: 10.2138/am.2009.3050 |
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
Wu T, Kong W, Zhang Y, Xing Z, Zhao J, Wang T, Shi X, Luo Y, Sun X 2019 small Methods 3 1900356 DOI: 10.1002/smtd.v3.11 |
| [19] |
Wu T, Zhu X, Xing Z, Mou S, Li C, Qiao Y, Liu Q, Luo Y, Shi X, Zhang Y, Sun X 2019 Angew. Chem. Int. Ed. 58 18449 DOI: 10.1002/anie.v58.51 |
| [20] |
Li B, Zhu X, Wang J, Xing R, Liu Q, Shi X, Luo Y, Liu S, Niu X, Sun X 2020 Chem. Comm. 56 1074 DOI: 10.1039/C9CC08971C |
| [21] |
|
| [22] |
Wang J, Tafen D N, Lewis J P, Hong Z, Manivannan A, Zhi M, Li M, Wu N 2009 J. Am. Chem. Soc. 131 12290 DOI: 10.1021/ja903781h |
| [23] |
|
| [24] |
|
| [25] |
Umebayashi T, Yamaki T, Itoh H, Asai K 2002 Appl. Phys. Lett. 81 454 DOI: 10.1063/1.1493647 |
| [26] |
|
| [27] |
Ali M, Hussain R, Tariq F, Noreen Z, Toufiq A M, Bokhari H, Akhtar N, ur Rahman S 2020 Appl. Nanosci. 10 1005 DOI: 10.1007/s13204-019-01193-0 |
| [28] |
|
| [29] |
Noorimotlagh Z, Kazeminezhad I, Jaafarzadeh N, Ahmadi M, Ramezani Z, Martinez S S 2018 J. Hazard. Mater. 350 108 DOI: 10.1016/j.jhazmat.2018.02.022 |
| [30] |
|
| [31] |
Yang G, Jiang Z, Shi H, Xiao T, Yan Z 2010 J. Mater. Chem. 20 5301 DOI: 10.1039/C0JM00376J |
| [32] |
|
| [33] |
Sim L C, Leong K H, Ibrahim S, Saravanan P 2014 J. Mater. Chem. A 2 5315 DOI: 10.1039/C3TA14857B |
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
Lu X H, Huang X, Xie S L, Zheng D Z, Liu Z Q, Liang C L, Tong Y X 2010 Langmuir 26 7569 DOI: 10.1021/la904882t |
| [44] |
Pham C V, Krueger M, Eck M, Weber S, Erdem E 2014 Appl. Phys. Lett. 104 132102 DOI: 10.1063/1.4870297 |
| [45] |
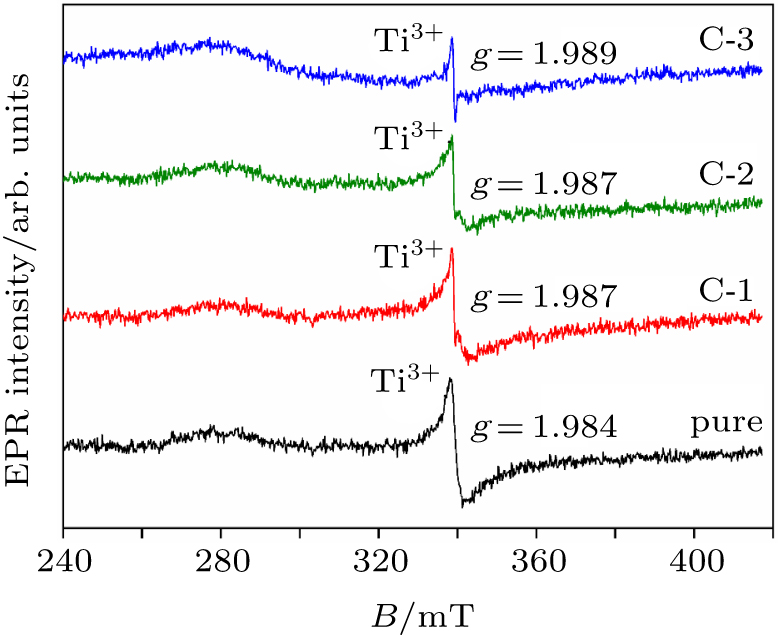
Lund A, Shiotani M, Shimada S 2011 Principles and applications of ESR spectroscopy New York Springer 91
|
| [46] |
Zhou S, Čižmár E, Potzger K, Krause M, Talut G, Helm M, Fassbender J, Zvyagin S, Wosnitza J, Schmidt H 2009 Phys. Rev. B 79 113201 DOI: 10.1103/PhysRevB.79.113201 |
| [47] |
Hurum D C, Gray K A, Rajh T, Thurnauer M C 2005 J. Phys. Chem. B 109 977 DOI: 10.1021/jp045395d |
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
Yoon S D, Chen Y, Yang A, Goodrich T L, Zuo X, Arena D A, Ziemer K, Vittoria C, Harris V G 2006 J. Phys.: Condens. Matter 18 L355 DOI: 10.1088/0953-8984/18/27/L01 |
| [52] |
|
 )
)