† Corresponding author. E-mail:
Two-dimensional (2D) organic nanomaterials are fascinating because of their unique properties and pentential applications in future optoelectronic devices. Polyaniline (PANI) has attracted much attention for its high conductivity, good environmental stability and unusual doping chemistry. We report on liquid-phase exfoliation of layered PANI films grown by electrochemical polymerization. Atomic force microscopy images demonstrate that few- or even mono-layer PANI nanosheets can be fabricated. The PANI nanosheets can be transferred onto a variety of surfaces, providing a promising route to their incorporation into a variety of devices for further studies and various applications.
Two-dimensional (2D) organic nanomaterials suitable for catalysts, energy storage, ultra-sensitive sensors and flexible optoelectronic devices have gained great attention due to their intriguing physical and chemical properties.[1–3] To date, two strategies have been adopted to fabricate 2D organic nanomaterials. One is the so-called “bottom-up” method in which monomers are confined into a surface polymerize to form 2D organic nanomaterials.[4–6] Success of this method often relies on the quality of expensive substrates, fabrication techniques and special conditions such as ultra-high vacuum which is highly energy consuming.[7,8] In addition, the transfer from the growth substrates to target surfaces often introduces residues and defects that will deteriorate their performances.[9,10] An alternative method is the “top-down” method based on the exfoliation of layered bulk material.[11,12] It is gaining more attention because it represents an extraordinarily versatile, potentially up-scalable and sustainable route to the production of a wide variety of 2D nanomaterials.[9,13,14] The approach also allows the easy transfer of the products onto a variety of surfaces. Layered materials with inter-layer van der Waals interactions are often the start point of exfoliation. Many 2D organic nanosheets have been thus obtained from metal-organic frameworks and covalent organic frameworks.[15,16] However, the exfoliations of layered materials consisting of conventional polymer chains are rarely reported. Polyaniline (PANI) is one of the most important conducting polymers that have applications in various electronic devices due to its high conductivity, good environmental stability and unusual doping/dedoping chemistry.[17–21] PANI with nano-lamellar structure is even more useful in electronic and opto-electronic devices because of its distinctive conducting pathways and surface interactions.[22,23] In this work, we seek to grow layered PANI with the popular electrochemical polymerization method and fabricate 2D PANI via liquid-phase exfoliation.
Aniline (99+%) was purchased from Alfa Aesar, and distilled prior to use. Sulfuric acid, acetone, methanol, and isopropanol were purchased from Beijing Chemical Reagents Co. and directly used without further purification. The layered PANI films were electrochemically polymerized using a CHI660A electrochemical workstation. The saturated calomel electrode (SCE) was used as the reference electrode, a silicon wafer and a polished platinum plate were used as the working electrode and counter electrode, respectively. The concentrations of aniline and sulfuric acid in the electrolytes were 0.1 M and 0.5 M respectively. The polymerization was carried out at 0.79 V versus SCE for 1000 s. The obtained PANI films were first washed using distilled water, then released by gentle solvent flow around the substrate with a micropipette. After being immersed in solvent for several days, sonication was adopted.
The scanning electron microscope (SEM) images were acquired using Hitachi S-4800. The x-ray reflectivity was measured by performing a θ–2θ scan on a Bruker D8-advance diffractometer, collecting diffraction peaks along the [001] direction. The rocking curve around the (001) peak was obtained by setting the 2θ angle at that of (001) peak and rocking the sample. The atomic force microscopy (AFM) images were obtained in ScanAsyst using a Bruker Multimode 8 AFM. The optical microscope images were obtained using an optical microscope (Nikon LV100ND).
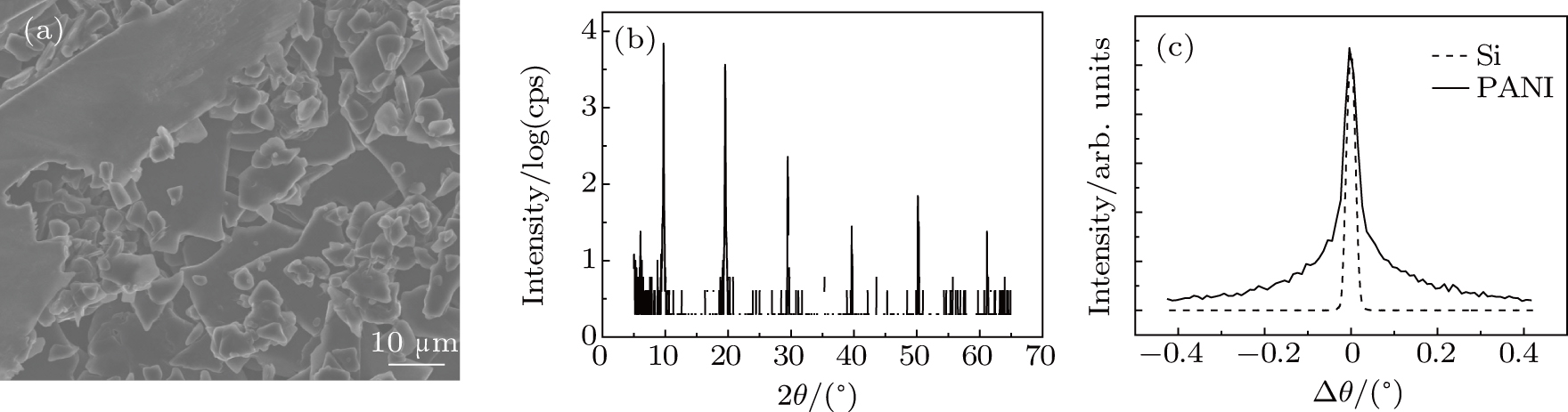
We adopt the electrochemical polymerization method, by which an extended chain conformation can be obtained,[24] to produce lamellar PANI crystals. When the potential is set to be beween 0.7 V and 0.8 V with resepct to the SCE, the polymerization goes directly towards linear PANI, favoring the formation of layered PANI crystals. The SEM image and x-ray reflection pattern (Figs.
The key to successful exfoliation is to find appropriate solvents. The commonly used solvents are 1-methyl-2-pyrrolidone (NMP) and dimethylformamide (DMF), which have proven useful to exfoliate van der Waals crystals down to thin sheets and monolayer sheets.[26,27] However, these solvents cannot be used to exfoliate PANI crystals because they are good solvents for PANI chains. Moreover, complex processes are needed to remove these highly toxic solvents after the exfoliation. We therefore use other common solvents to exfloliate the layered PANI.
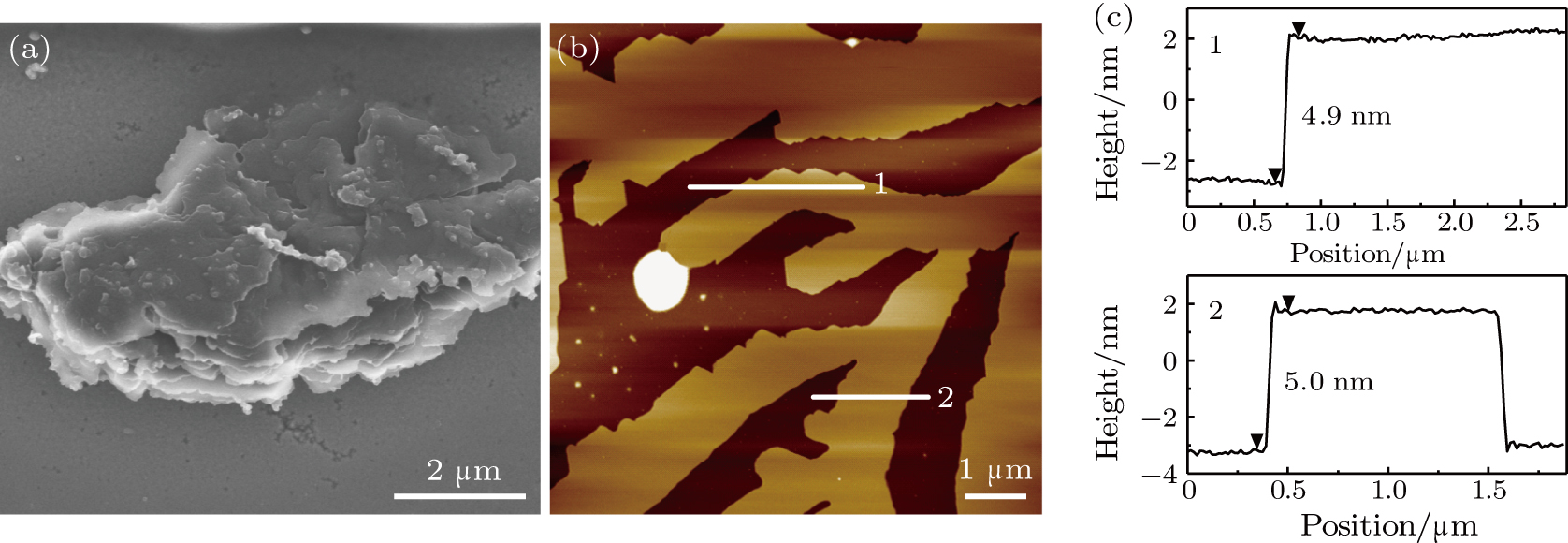
Acetone has been proved to be a suitable solvent to induce exfoliation.[28] It is one of the most common solvents with low toxicity and strong volatility, which is convenient for removing the solvent. We immerse the films in acetone for several days to swell them. After swelling, the films show loose laminar structures as shown in Fig.
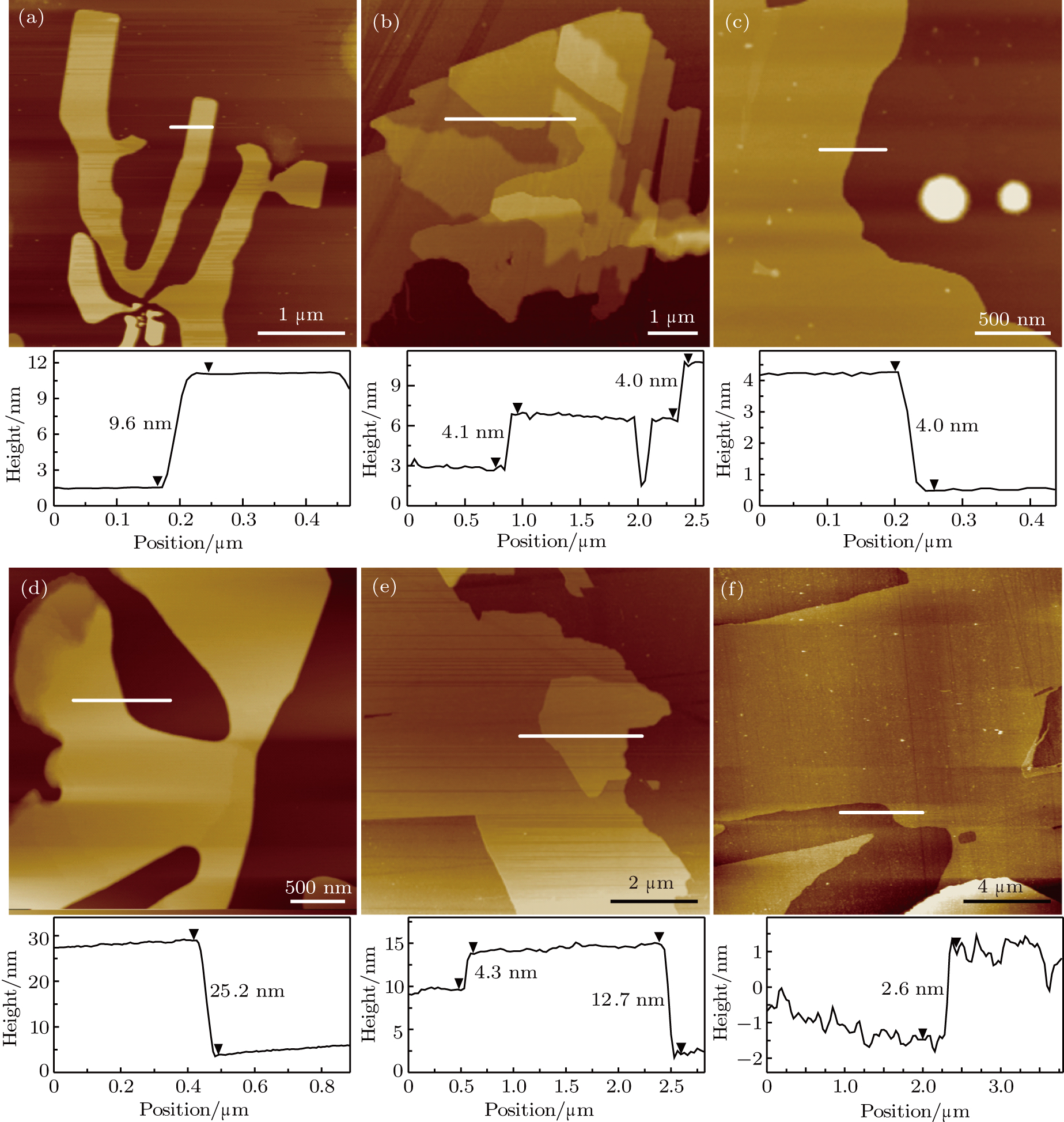
We test two other common solvents (isopropanol and methanol) we sorted out according to the Hansen solubility parameter theory,[29] and survey their ability to exfoliate PANI films under the same condition as that in Fig.
Besides the solvents, sonication conditions also play an important role in determining the thickness and lateral dimensions of the nanosheets.[13,33] We perform additional experiments by adjusting the sonication time in different solvents in order to understand the factors that determine the quality of the nanosheets. Figure
To see whether it is possible to push exfoliation forward to obtain single-layer PANI, we increase the sonication time and power. With the increase of time to 40 min at 100 W (Fig.
The above results illustrate that not only the solvent, but also the sonication power and time have a significant effect on the number of layers of the two-dimensional PANI (See the following Table
| Table 1.
Effects of sonication power (P) and time (T) on the number of layers (L) of 2D PANI. . |
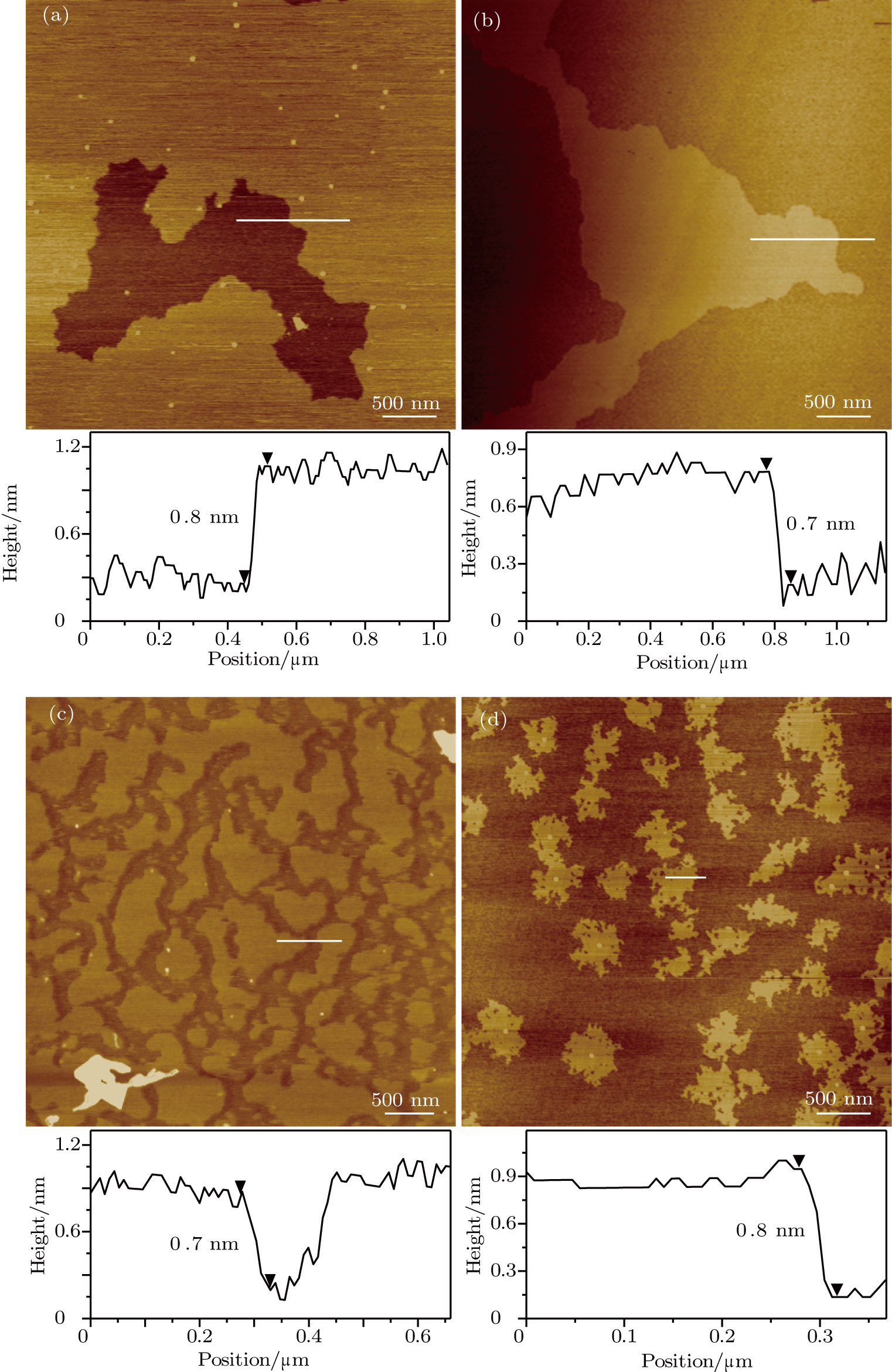
It is noted that an advantage of the PANI nanosheets formed by liquid exfoliation is that they are transferable onto various substrates, providing a promising route to the incorporation of the PANI nanosheets into a variety of devices for further studies and various applications. We transfer the PANI nanosheets onto mica, highly oriented pyrolytic graphite (HOPG), Si


 | Fig. 6. (color online) AFM images and the corresponding height profiles of PANI nanosheets transferred onto HOPG (a), Si

|
In this work, we prepare 2D PANI nanosheets with various lateral sizes and thickness values through liquid-phase exfoliation of layered PANI films grown by electrochemical polymerization. We demonstrate that common solvents, such as acetone and isopropanol, are good solvents for preparing the 2D organic nanomaterials. Unlike previously reported solvent (NMP or DMF), these solvents are more environmentally friendly and can be easily removed through drying at room temperature. This provides a novel way of fabricating the 2D PANI nanosheets. We also show that these nanosheets can be transferred onto a variety of surfaces, which is an important step for applications.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] |