Project supported by the National Natural Science Foundation of China (Grant No. 51262032).
Project supported by the National Natural Science Foundation of China (Grant No. 51262032).
† Corresponding author. E-mail:
Single crystalline samples of type-I and type-VIII Ba8Ga16−xCuxSn30 (x = 0, 1) clathrates are prepared by the Sn-flux method. Effects of Cu-doping on stability and electrical properties of Ba8Ga16Sn30 single crystal are explored by first-principle and experiment. All samples are heated to different high temperatures and maintained at these temperatures for 120 min and then cooled to room temperature to explore their structural stabilities. Results from DTA and powder x-ray diffraction analysis indicate that type-I Ba8Ga16Sn30 structure is transformed into type-VIII phase after the sample has been heated to 185 °C. Type-VIII BGS is stable during heating and cooling, but type-VIII Ba8Ga15CuSn30 decomposes into Sn and Ba(Ga/Sn)4 during cooling. Meanwhile, the electrical properties of type-I samples are measured, their electrical conductivities are enhanced, and the Seebeck efficient is reduced with Cu substitution. The type-I samples after phase transformations show the electrical characteristics of type-VIII samples.
Thermoelectric materials are attracting considerable attention because they can directly convert waste heat into electrical energy.[1] The thermoelectric efficiency of a material is determined by its figure of merit ZT = α2σT/κ, where α is the Seebeck efficient, σ is the electrical conductivity, and κ is the thermal conductivity.[2] Thus, low lattice thermal conductivity in combination with reasonable electrical conductivity is the most important requirement to obtain excellent thermoelectric properties.
The phonon-glass property of the semiconducting clathrate compound, which satisfies the “phonon glass-electron crystal” concept,[3] determines the candidate position of promising thermoelectric material. Many types of clathrates are available, among which type-I and type-VIII of Si-, Ge-, and Sn-based thermoelectric clathrates have drawn significant attention,[4–7] the Sn-based clathrates can be potentially used in thermoelectric electricity generation because of their low lattice thermal conductivities and high Seebeck efficients.[8–11]
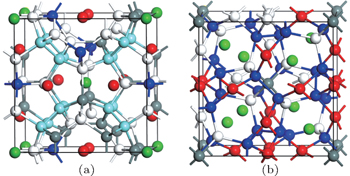
Ba8Ga16Sn30 (BGS) is a dimorphic compound with type-I and type-VIII structures.[9,10] The unit cell of type-I clathrate (space group 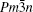
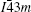
BGS and Sn-based clathrate with elemental partial substitution for Ga in the framework of BGS possesses high values of ZT.[17–21] Saiga et al.[17] enhanced the ZT values of p- and n-type type-VIII BGS up to 1.0 and 0.9 at 480 K, respectively, using optimized carrier concentrations of samples. Meng et al.[18] synthesized single crystal VIII-Ba8Ga16−xGexSn30 by partial substitution of Ge for Ga, electrical conductivity was enhanced by 62% through doped Ge, and obtained the maximum ZT value of 1.25 when x = 0.5 at around T = 500 K. Deng et al.[19] doped Cu into type-VIII Ba8Ga16−xCuxSn30 at a very low level of x (0.033), which resulted in the highest ZT of 1.35 among the ZT values of the n-type substituted BGS samples. Thus, the ZT values for the n-type BGS were enhanced by partial substitution of Ge or Cu for Ga in the framework. Moreover, Du et al.[22] recently found that type-I Ba8Ga16Sn30 is stable only in a certain temperature range, and this material transforms into type-VIII Ba8Ga16Sn30 when heated to 739 K. However, Saiga et al.[23] reported that Ba8Ga16Sn30 with type-I structure changes into type-VIII when heated above 520 K. Also, our previous work found that the syntheses at different reactive temperatures lead to different types of clathrates.[24] The thermoelectric materials are usually used at high temperature. Thus, more accurate information about structure stability under high-temperature and the evolution of the thermoelectric property based on temperature is extremely important for practical application. Furthermore, doping can usually increase effectively the ZT value, however, little attention has been paid to the effect of doping on structure stability. Therefore, in the present work, the structure stabilities for type-I and type-VIII Ba8Ga16Sn30 are systematically examined, and the influence of Cu doping on the structure stability is explored. Finally, the evolutions of electrical conductivity and thermoelectric property of samples based on temperature are analyzed.
The Sn-flux method was adopted to prepare single-crystalline samples of Ba8Ga16−xCuxSn30. High-purity elements Ba (ingot, 99.9%), Ga (ingot, 99.999%), Sn (ingot, 99.999%), and Cu (ingot, 99.999%) were weighed according to the atomic ratio of Ba:Ga:Cu:Sn = 8:16–x:x:50 (x = 0, 1). Type-I and type-VIII samples were synthesized by heating the mixture, which was sealed in an evacuated quartz tube and heated, as follows: for x = 0, 490 °C and 500 °C; for x = 1, 540 °C and 560 °C. The mixture was maintained for 10 h and then slowly cooled over 50 h to 390 °C.[24] The quartz tube was removed from the furnace at 390 °C. The tube was then centrifuged to separate the crystals from the molten Sn solvent.
Phase was characterized by powder x-ray diffraction (XRD, Bruker D8 Cu Kα). Thermal stability was measured by thermogravimetry–differential thermal analysis (TG-DTA; STA449F3 Jupiter). Electron probe microanalysis (EPMA, JXA-8230) was used for chemical composition analysis. Electrical conductivity σ and Seebeck efficient α were measured at temperatures ranging from 25 °C to 250 °C. Comparison was performed using constantan (Ni: 40%) as a reference sample with a known α(T) to measure the Seebeck efficient α. The σ(T) values of the samples were measured by the DC current method, with the DC current kept at 20 mA. All measurements were performed in a vacuum to avoid oxidation.
The crystal sizes of type-I single crystals are about 4 mm. By contrast, the type-VIII single-crystal samples obtained are approximately 7 mm in diameter. As shown in Fig.
 | Fig. 2. As-grown single crystals of (a) type-I and (b) type-VIII BGCS clathrate, (a) type-I BGCS and (b) type-VIII BGCS. |
Thermostabilities of all samples are explored by performing DTA and XRD for pony-size (2 mm) samples. The samples are heated to 490 °C and then slowly cooled to 30 °C after having been maintained for 120 min. The heating and cooling rates are kept at 2 °C/min. To minimize the effect of the composition of a sample on the properties, all types of samples are cut from the corresponding same single crystal respectively.
The heating curve of type-I BGS in Fig.
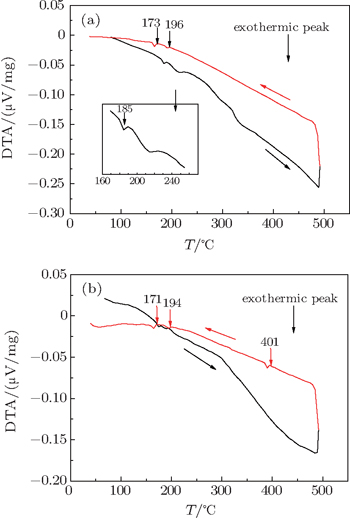 | Fig. 3. Differential thermal analysis (DTA) curves for (a) type-I BGS and (b) type-I BGCS heated to 490 °C. |
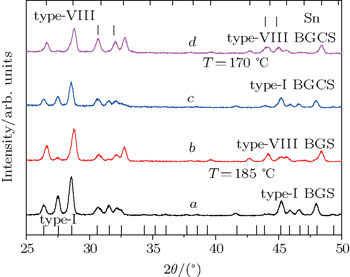 | Fig. 4. Powder x-ray diffraction patterns for type-I BGSs before (a) and after (b) being heated to 185 °C, and type-I BGCSs before (c) and after (d) being heated to 170 °C. |
No additional peak is found on the cooling curve of type-I BGS in Fig.
No peak is found on the heating and cooling curves of the type-VIII BGS heated to 490 °C (Fig.
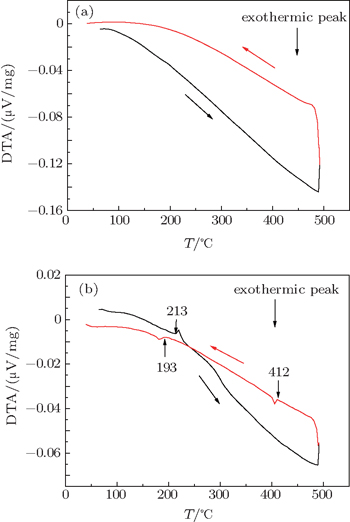 | Fig. 5. Differential thermal analysis (DTA) curves for (a) type-VIII BGS heated to 490 °C and (b) type-VIII BGCS heated to 490 °C. |
Type-I and type-VIII BGS are also heated to 550 °C. Figure
 | Fig. 6. Differential thermal analysis (DTA) curves for (a) type-I BGS and (b) type-VIII BGS heated to 550 °C. |
This phenomenon causes the chemical composition of the molten state to deviate from the ratio of 8Ba:16Ga:30Sn, and consequently, the melting type-I and type-VIII clathrate transform into type-VIII and type-I, respectively.
The formation energy Ef and binding energy E0 of the system are calculated using the first-principle to elucidate the effect of Cu doping on the structural stability. Formation energy is defined as the total energy difference between the compound and its corresponding isolated bulk constituent elements.[25] The calculated method and results have been reported in our previous work,[24] and the Ef and E0 are increased with Cu doping, which agree with other theoretical work.[26,27] Thus, Cu doping will reduce the stabilities of I- and VIII-BGS, so the phase transition temperature of I-BGCS is lower than that of I-BGS.
Milliken population for the series is obtained for further revealing the effects of Cu-doping on structure of I- and VIII-BGS. As shown in Table
| Table 1. Orbit populations and valence charges for the series. . |
Overlap populations and bond length for the series are analyzed. The results are shown in Table
| Table 2. Overlap populations and bond lengths for the series. . |
We have previously explored the electrical properties of type-VIII Ba8Ga16−xCuxSn30 single crystals, and Cu doping significantly improving the conductivity of type-VIII BGS;[19] our experimental results revealed that the electrical properties of the type-VIII phase have a negligible variation after the high-temperature heating process.[24] Hence, the present paper aims to investigate the effects of the high-temperature heating process on the electrical and thermoelectric property of type-I phase, and the evolutions of electrical conductivity and thermoelectric property of type-I samples based on temperature are investigated.
The plots of electrical conductivity σ of type-I samples versus temperature on heating and cooling between 25 °C and 250 °C are shown in Fig.
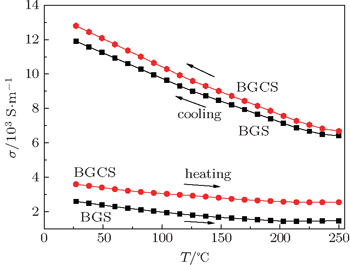 | Fig. 8. Plots of electrical conductivity σ of the type-I samples versus temperature on heating and cooling between 25 °C and 250 °C. |
The Seebeck efficient α of all single crystals samples are negative (Fig.
 | Fig. 9. Plots of Seebeck efficient α of the type-I samples versus temperature on heating and cooling between 25 °C and 250 °C. |
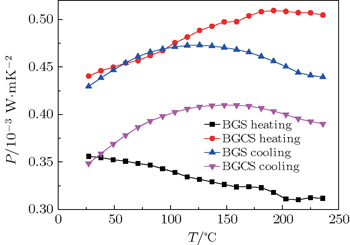
The temperature dependences of the power factor for type-I single crystals are shown in Fig.
The structural stabilities and electrical properties of Cu-substituted Ba8Ga16−xCuxSn30 single crystals are explored by first principle and experiment. The theory indicates that Cu doping will reduce the stabilities of I- and VIII- Ba8Ga16Sn30. The DTA curve and powder XRD results indicate that type-I BGS transforms into type-VIII above 185 °C, and that the critical transform temperature of type-I BGCS is lower than that of type-I BGS. Type-VIII BGS is stable during heating and cooling. However, type-VIII BGCS decomposes into Sn and Ba(Ga/Sn)4 during cooling. The molten state of type-I BGS can transform directly into type-VIII BGS, and the melting type-VIII BGS can transform into type-I phase on cooling. The measured electrical properties suggest that Cu doping enhances the electrical conductivity of type-I BGS. However, the absolute value of the Seebeck efficiency decreases. The type-I sample after phase transformation exhibits type-VIII characteristics. After heating treatment, the power factor of BGS is improved, in contrast, the P of BGCS is reduced due to the variations of α and σ.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 |