† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11005143, 11405259, and 51274046) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China (Grant No. [2014]1685).
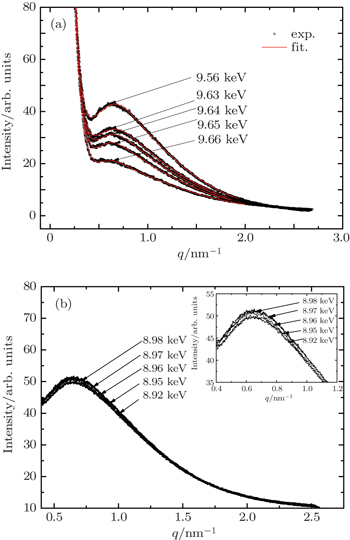
In the present work, the precipitate compositions and precipitate amounts of these elements (including the size distribution, volume fraction, and inter-precipitate distance) on the Cu-containing 7000 series aluminum alloys (7150 and 7085 Al alloys), are investigated by anomalous small-angle x-ray scattering (ASAXS) at various energies. The scattering intensity of 7150 alloy with T6 aging treatment decreases as the incident x-ray energy approaches the Zn absorption edge from the lower energy side, while scattering intensity does not show a noticeable energy dependence near the Cu absorption edge. Similar results are observed in the 7085 alloy in an aging process (120 °C) by employing in-situ ASAXS measurements, indicating that the precipitate compositions should include Zn element and should not be strongly related to Cu element at the early stage after 10 min. In the aging process, the precipitate particles with an initial average size of ∼ 8 Å increase with aging time at an energy of 9.60 keV, while the increase with a slower rate is observed at an energy of 9.65 keV as near the Zn absorption edge.
Nanoscale precipitates of trace elements play an important role in the microstructures and performances in alloys,[1] and qualitative analyses of precipitate composition and quantitative evolution of microstructures become very necessary. Over the years, a lot of studies have focused on the nanoscale precipitates of aluminium alloys with different technologies. Transmission electron microscopy (TEM) and tomographic atom probe (TCP) can give clear information about the shapes and sizes of precipitates. However, these two methods have the disadvantages of the lower statistical analysis and inconvenience for the in-situ study in the aging process. Small-angle x-ray scattering (SAXS) enables the obtaining of many important parameters of precipitates including particle shape and length scale, so it has been widely used for characterizing the nanoscale microstructure of alloys.[2–8] When SAXS measurements are performed at different energies across the absorption edge of one of the elements present in the microstructure, we should give access to information about the composition difference between the precipitate and matrix in this specific element. Such measurements are called anomalous small-angle x-ray scattering (ASAXS), It is sensitive to the anomalous dispersion effect of a specific element.[9,10] The use of several methods could prove compositive information and complement each other.
Alloys in the Al–Zn–Mg–Cu system (7xxx series) have been extensively used as high-strength structural materials such as in the aerospace and automotive industries. The precipitate characteristics and mechanism have been investigated to probe the relationship between the precipitates and applied properties of Al alloy. TEM investigations reported that smaller clusters were found at 7050 alloy after a short aging time at 121 °C, while larger precipitates were observed with increasing aging time.[11] SAXS measurements[6,12] were carried out on the Al–Zn–Mg–Cu alloy (7150 and 7108.5) with different aging treatments, showing the evolutions of the volume fraction and particle size during aging–retrogression–reaging treatment. SAXS investigations also suggested that there are two types of particles with different sizes (smaller Guinier Preston (GP) zone and larger η′ particles) in 7xxx series Al alloys aged at temperatures in a range between about 60 °C and 100 °C.[13] Positron annihilation spectroscopy investigations indicated that the Mg-vacancy, or Zn-vacancy or Zn2-vacancy complex, has a significant effect on GP zone formation.[14,15] The three-dimensional (3D) atom probe analyzed by Sha and Cerezo[11] indicated that the precipitates of Zn are accompanied by Mg, while Mg-rich clusters were observed at the early-stage. They have also proposed that smaller clusters growing into blocky GPI zones at the earliest stages of aging (< 30 min) would be related to the more Mg elements in the clusters, while the Zn-rich is a probable reason of some smaller clusters becoming elongated and subsequently growing into platelet η′ after 30 min. Despite the fact that there are a lot of previous studies, our understanding of precipitation is still limited, and especially the quantitative information is lacking at early-stage during the in-situ probing size, density and chemistry. In this study, we are employing ASAXS to probe the precipitates on Cu-containing 7000 series aluminum alloys (7150 and 7085Al alloys), provide more information about the precipitate compositions for the investigation of the formation mechanism.
Two types of 7000 series aluminum alloys (7150 and 7085Al alloys) were used in this study. A 7150 aluminum alloy plate with compositions (mass fraction) of Zn 6.56%, Mg 2.25%, Cu 2.10%, Zr 0.12%, was prepared when the solution was heat-treated first at 470 °C for 90 min and then 480 °C for 60 min, cold water quenching, followed by the typical T6 treatment (120 °C for 24 h). A 7085 aluminum alloy plate was a hot-rolled aluminum alloy plate, with compositions (mass fraction) of Zn 7.5%, Mg 1.7%, Cu 1.4%, Zr 0.12%, and Al balance. Specimens of 7085 alloy cut from the alloy plate were treated by solution at 475 °C for 120 min, cold water quenching, followed by pre-aging at 120 °C for 12 h. Here the solution treatment was carried out in order to dissolve the second phase particles (formed during casting and/or rolling) into the solid solution adequately.
SAXS experiments were performed on the BL16B1 beamline of Shanghai Synchrotron Radiation Facility (SSRF). 2D SAXS patterns were recorded by Mar165 CCD with a pixel size of 80 μm × 80 μm. Prior to the experiments, the specimens were mechanically polished into foils of about 100 μm in thickness. A sample-to-detector distance of ∼ 2 m was chosen, giving access to a range of scattering vector Q [0.15 nm−1 ∼ 3 nm−1]. During the experiments, the beam size was 2 mm × 1 mm. The temperatures of the specimens were monitored by using a Linkam thermal stage THMS600 (Linkam Scientific Instruments). In-situ SAXS measurements on 7085 alloy were carried out during aging at a temperature of 120 °C. During the whole heat treatment, the temperature uncertainty is within ±0.2 °C. In the present work, one-dimensional integrated intensity curves were obtained from 2D SAXS patterns by employing Fit2D software, while SAXS data quantitative data analyses were conducted using the Irena curve fitting package within IGOR Pro.[16] In the model, a form factor of spheroid and a structure factor of a type of precipitate were chosen, and a logarithmic normal size distribution was used to account for the polydispersity. The mean radius, distance, size distribution of precipitates, etc. would be obtained.
Figure
In each SAXS profile of Fig.
| Table 1. Structural parameters obtained from fitting for 7150 Al alloy after T6 treatment, obtained at various energies below the Zn absorption edge. . |
Figure
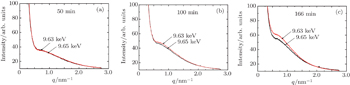 | Fig. 4. SAXS profiles obtained at different energies (9.63 keV and 9.65 keV) and different stages (50 min (a), 100 min (b), and 166 min (c)) for the 7085 alloy during aging at 120 °C. |
Figure
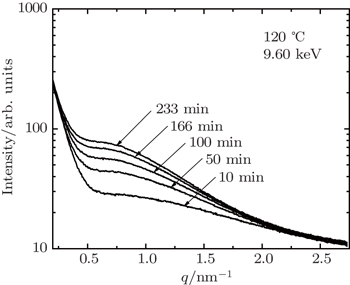 | Fig. 5. SAXS profiles obtained during aging at a temperature of 120 °C for 7085 alloy. The incident energy of 9.60 keV is below Zn absorption edge. |
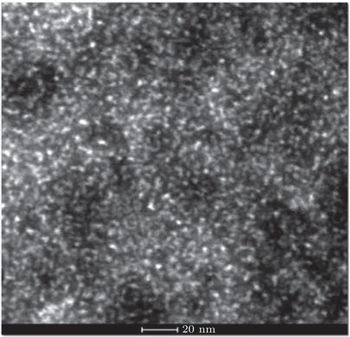
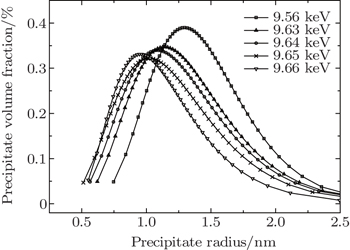
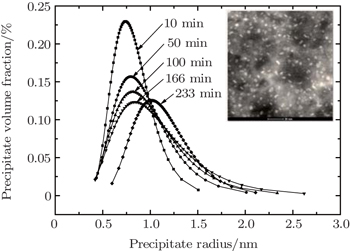 | Fig. 6. Variations of distribution of volume fraction with precipitate size for the 7085 alloy during aging at 120 °C. The inset shows bright-field TEM micrograph for 7085 alloy after aging. |
In Fig.
We investigate Al alloys of 7150 and 7085 at energies near the Zn and Cu absorption edge using ASAXS. We find that scattering intensity decreases as the incident x-ray energy approaches the Zn absorption edge from the lower energy side, while the scattering intensity does not show a noticeable energy dependence near the Cu absorption edge. The in-situ ASAXS probing during aging at 120 °C, shows that the SAXS profile obtained near the Zn absorption can be distinguished after 50-min aging, which indicates that the more Zn-rich smaller clusters could be elongated and subsequently grow into platelet η′ after 50 min.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 |