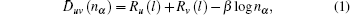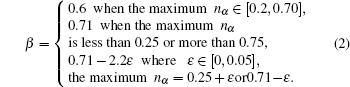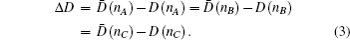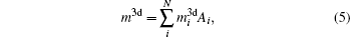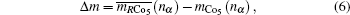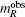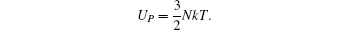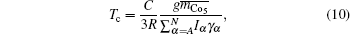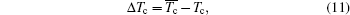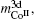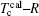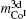1. IntroductionOwing to the large magnetocrystalline anisotropies, high Curie temperatures and high saturation moments at room temperature, RCo5 (R = rare earth) intermetallic compounds have received considerable scientific and technological attention in the past decades,[1,2] and the studies have covered the experiment, theory and technology for developing the advanced rare earth permanent magnetic materials.[3–5] The study on magnetic properties of RCo5 intermetallic compounds is derived from the magnetization measurements on single crystals.[6] Rao and Greedan have established the relationships between the basic parameters in crystal-field theory and the magnetocrystalline anisotropy in most RCo5 intermetallic compounds.[7] The single-ion anisotropy has been found to have a fundamental influence on the behavior of the magnetic system and the related mechanism has been studied.[8,9] In many cases, the single-ion anisotropy model contains many calculating parameters especially in the Heisenberg model proposed based on the exchange coupling interaction. As for the first principle theory, the magnetic properties of RCo5 compounds are usually discussed within a two-sublattice model consisting of R and Co sublattices.[10,11] The computational methods based on the density functional theory (DFT) provide an important insight into the electronic structure and the related physical properties for the various materials. The basic DFT approximation (local spin density approximation-LSDA) is usually restricted to the so-called open core treatment of the 4f electrons.[12,13] However, LSDA could not predict the energetics of the various magnetic orders due to the absence of the energy gap between the filled and the empty states and underestimations of the orbital magnetic moments.[14,15] The theoretical work on the anisotropy and Curie temperature of R–(Fe,Co) magnetic alloys were carried out in the molecular-field approximation by studying the exchange coupling interactions between the 3d electrons of Fe or Co and 4f electrons of rare earth.[16] However, there are too many theoretical parameters in the complicated calculation. Also, the samples should be of the single-crystal.[17]
As for the first principle theory, it could not initially be used to study the magnetism of rare-earth-based intermetallic compounds. In recent years, a framework of the LSDA + U approach has been developed to calculate the magnetic properties of rare earth alloys and compounds based on the mean-field theory.[18] However, the calculation is restricted by the structural cell model and the capacity of the computer. In the present paper, we use the empirical electron theory of solids and molecules (EET), which is based on Pauling’s electron theory of metals.[19] Owing to its lesser computing parameters than the molecular-field and the first principle theories, the model is simpler than the theories of crystal-field and first-principle without considering any integral or differential calculation. The EET can be used to investigate the correlations between the valence electronic structures and multiple physical properties of solid state materials. The approximations, which are based on the electronic structures, can work in studying the essential mechanisms of physical phenomena as reported. On one hand, the electronic structure analyses could reveal many natures of physical phenomena such as: orbital hybridization, band gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), vertical ionization potentials, and electron affinities, which are important for studying the correlations between the electronic structures and physical properties in molecules and solids.[20–22] The multiple properties such as cohesive energy, melting point, transition temperature, magnetic moment, Curie temperature, superconductor temperature, etc. have been calculated based on the electron states in the electronic structure.[23–26] In this study, the magnetic moments and Curie temperatures of RCo5 (R = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Er) intermetallics are systematically studied with EET and the relations between the electronic structures and magnetic characteristics of these compounds are discussed as well.
2. Empirical electron theory (EET)EET is a semi-empirical theory based on the Pauling valence-bonding theory and Hume–Rothery electron concentration rule. As a supplement, the equivalence valence electron hypothesis is introduced for the application of the electron theory to the crystal structures. The theory consists of three general hypotheses and one method of calculating bond distances. Hypothesis 1: the atoms in a solid and a molecule are in general hybridization of the two states, which can be called the h (head) and t (tail) states. Both states have one or two kinds of valence electrons; that is, covalent and newly introduced lattice electrons. Hypothesis 2: the hybridization of the two states is discontinuous, allowing only certain relative compositions to be calculated by a k-equation. Hypothesis 3: the covalent bond length between atom u and v with the number of covalent electron pairs denoted by nα is related by
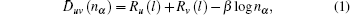
where
Ru(1) and
Rv(1) denote the radii of single-bond for u and v atoms, respectively. The parameter
β can reflect how much the electron clouds of the u and v atoms overlap each other, and
β values are 0.6 for metals and alloys and 0.71 for the other nonmetal compounds, respectively. Here a modification of Pauling’s theory is made for (
n − 1) value according to the maximum of
nα (
α =
A,
B,
C,…,
N), and the (
n − 1) values are listed below.
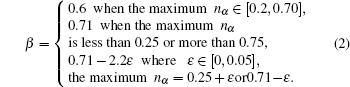
The bond length difference (BLD) analytical method: the method is trying to find the derivations (Δ
D) between the theoretical
D(
nα) and experimental bond lengths
D(
nα) until Δ
D ≤ 0.05 Å. For calculating the theoretical bond lengths
D̄(
nα)(
α =
A,
B,
C,…
N), the
nα (
α =
A,
B,
C,…,
N) values should be first calculated based on (
α =
B,
C,…,
N) experimental bond length difference [
D(
nA) −
D(
nα)] equations as reported in Ref. [
17]. The theoretical bond lengths
D(
nα) could be calculated by substituting the
nα (
α =
A,
B,
C,…,
N) values into Eq. (
1).
From the derivation, it is seen that
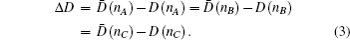
Hence the difference Δ
D is a criterion for all the
D̄(
nα)’s, that is, the theoretical explanation for all the
D̄(
nα)’s of a crystalline or molecular structure. If it is smaller than 0.05 Å, it proves that the valence bond electron distribution is correct in the first order approximation. However, the only criterion limitation of Δ
D is not enough to determine the electronic structure. Thus, the extra physical property criterions, such as melting point, cohesive energy, magnetic moments are necessary. Here the moment and Curie temperature criterions are discussed.
3. Calculations of magnetic moment and Curie temperature3.1. Magnetic moments[23]According to the criterion of ΔD ≤ 0.05 in the BLD method, the hybridization states of the electrons in solid and molecules could be determined, and the relative values of Chσ and Ctσ are determined.
Then there is given the equation for calculating the magnetic moments:

For a molecule, we then have
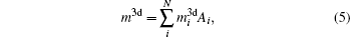
where
Ai and

denote the atom number of the
i-th atom and its 3d magnetic electron number. The Δ
m is also considered as a criterion for all the
m3d for the Co atoms at 2
c and 3
g sites, and expressed as
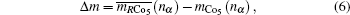
with

where the
mCo5(
nα) and
mCo5 (
nα) are corresponding to the theoretical and observed magnetic moments.
The magnetic moments of rare earth 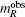 are the theoretical moments in ordered status gμBJ (J is angular momentum). The moment contributed by 4f electron of rare earth depends on 4f electron spin alignment at R sublattice, and its values are positive for light rare-earth- (LRE) based RCo5 compounds and negative for the heavy rare-earth- (HRE) based RCo5 compounds, respectively. Therefore, the average magnetic moment per atom is
are the theoretical moments in ordered status gμBJ (J is angular momentum). The moment contributed by 4f electron of rare earth depends on 4f electron spin alignment at R sublattice, and its values are positive for light rare-earth- (LRE) based RCo5 compounds and negative for the heavy rare-earth- (HRE) based RCo5 compounds, respectively. Therefore, the average magnetic moment per atom is

About all, the criterion Δ
m gives another limitation for estimating the calculations.
3.2. Curie temperatureThe model for calculating Curie temperature is based on the assumption of molten ferromagnetic order structure by the heat vibrations induced by the phonon energy with increasing temperature. When the temperature reaches a certain value of Tc, the phonon energy equals the magnetic energy. If T > Tc, the magnetic order is molten and the ferromagnetic characteristics disappear. This transition temperature is regarded as the Curie temperature. The energy of N phonons is
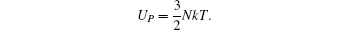
The average magnetic energy per bond is
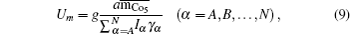
where
Up and
Um denote the phonon and magnetic energy, respectively. When
Up =
Um, and
g = 2.2 for Co, then we have
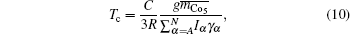
where
R = 8.31 J/mol, and
C = 1.542 × 10
4.
Then here is given the criterion, ΔTc.
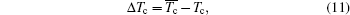
where the
Tc and
Tc denote the calculated and observed Curie temperature respectively.
In summary, the theoretical D (nα)’s should fit to all the limitations of ΔD, Δm, and ΔTc well, and then the relations between the magnetic characteristics and electronic structures in the exact hybridization can be revealed. The calculated results are listed in Tables 1–3.
Table 1.
Table 1.
 Table 1. Lattice parameters of RCo5 and valence bond lengths. .
| RCo5 |
YCo5 |
LaCo5 |
CeCo5 |
PrCo5 |
NdCo5 |
SmCo5 |
GdCo5 |
TbCo5 |
DyCo5 |
ErCo5 |
| a/Å |
4.951 |
5.120 |
4.920 |
5.012 |
5.010 |
5.002 |
4.975 |
4.961 |
4.924 |
4.885 |
| c/Å |
3.975 |
3.980 |
4.029 |
3.990 |
3.960 |
3.964 |
3.974 |
3.981 |
3.986 |
4.002 |
| R–CoI/Å |
2.859 |
2.956 |
2.840 |
2.894 |
2.893 |
2.888 |
2.872 |
2.864 |
2.843 |
2.820 |
| R–CoII/Å |
3.175 |
3.242 |
3.179 |
3.203 |
3.193 |
3.191 |
3.183 |
3.180 |
3.168 |
3.158 |
| CoI–CoII/Å |
2.448 |
2.479 |
2.465 |
2.465 |
2.452 |
2.452 |
2.451 |
2.452 |
2.448 |
2.448 |
| Table 1. Lattice parameters of RCo5 and valence bond lengths. . |
Table 2.
Table 2.
 Table 2. Calculation results of magnetic properties. In this table, nα (max) denotes the maximum number in the covalent electron pairs nα (α = A,B, … N), and the symbol “–” refers to the negative moment originating from the heavy rare earths since their moment arrangements are anti parallel to the direction of 3d moment of Co. .
| RCo5 |
YCo5 |
LaCo5 |
CeCo5 |
PrCo5 |
NdCo5 |
SmCo5 |
GdCo5 |
TbCo5 |
DyCo5 |
ErCo5 |
| nα (max) |
0.7087 |
0.6853 |
0.5693 |
0.6096 |
0.6251 |
0.6712 |
0.6642 |
0.6378 |
0.6352 |
0.7389 |
| |ΔD|/Å |
0.0074 |
0.0341 |
0.0129 |
0.0250 |
0.0386 |
0.0321 |
0.0297 |
0.0254 |
0.0325 |
0.0221 |
| (m3d/R)/μB |
0 |
0 |
2.37 |
3.2 |
3.45 |
1.32 |
–7.81 |
–9.4 |
–10.7 |
–9.4 |
| (m3d/CoI)/μB |
1.9628 |
1.8837 |
1.4047 |
1.9675 |
1.7120 |
1.9157 |
1.9628 |
1.7241 |
1.9628 |
1.9675 |
| (m3d/CoII)/μB |
1.4047 |
1.2876 |
0.7421 |
0.9457 |
0.8936 |
0.5707 |
1.7120 |
1.7120 |
1.9157 |
1.4872 |
| mCo5/μB |
8.1398 |
7.6303 |
7.4058 |
9.9716 |
9.5547 |
6.8634 |
1.2516 |
–0.8158 |
–1.0273 |
–1.0034 |
| mCo5/μB |
8.2 |
7.3 |
7.4 |
10.0 |
9.5 |
6.8 |
1.2 |
0.8 |
1.1 |
1.28 |
| |Δm/mCo5|/% |
0.73 |
4.52 |
0.08 |
0.28 |
0.58 |
0.93 |
4.30 |
1.98 |
6.61 |
21.61 |
| Tc/K |
881.15 |
854.14 |
717.96 |
937.38 |
927.40 |
769.13 |
1007.08 |
942.93 |
969.82 |
930.61 |
| Tc/K |
977 |
840 |
737 |
912 |
910 |
1020 |
1008 |
980 |
966 |
986 |
| |ΔTc/Tc|/% |
9.81 |
1.68 |
2.58 |
2.78 |
1.91 |
24.57 |
0.09 |
3.78 |
0.40 |
5.62 |
| Table 2. Calculation results of magnetic properties. In this table, nα (max) denotes the maximum number in the covalent electron pairs nα (α = A,B, … N), and the symbol “–” refers to the negative moment originating from the heavy rare earths since their moment arrangements are anti parallel to the direction of 3d moment of Co. . |
Table 3.
Table 3.
 Table 3. Analyses of valence electronic structures of RCo5. In this table, nc, nl, m3d, and nT denote the numbers of the covalent electrons, the lattice electrons, the magnetic electrons, and the total number of the electrons, respectively; σ is the number of quantum states. The rare earth element has a piece of the hybridization table, which possesses 10 kinds of quantum states. Element Co has 8 pieces of the hybridization table (A1, A2, B, C1, C2, D, E1, E2), and each of them records 18 kinds of quantum states. .
| RCo5 |
YCo5 |
LaCo5 |
CeCo5 |
PrCo5 |
NdCo5 |
SmCo5 |
GdCo5 |
TbCo5 |
DyCo5 |
ErCo5 |
| σ |
R |
3 |
1 |
1 |
3 |
1 |
1 |
1 |
1 |
3 |
3 |
| CoI |
E19 |
B11 |
E112 |
D14 |
A111 |
E110 |
E19 |
B12 |
E19 |
D14 |
| CoII |
E112 |
B14 |
A210 |
E27 |
B15 |
A211 |
A111 |
A111 |
E110 |
E111 |
| Ch |
CoI |
0.6543 |
0.6279 |
0.4682 |
0.4873 |
0.5707 |
0.6387 |
0.6543 |
0.5747 |
0.6543 |
0.4837 |
| CoII |
0.4682 |
0.4292 |
0.7421 |
0.9456 |
0.2979 |
0.5707 |
0.5707 |
0.5707 |
0.6387 |
0.4957 |
| nc |
CoI |
5.6914 |
4.7442 |
6.0737 |
0.0652 |
5.1880 |
5.7228 |
5.6914 |
4.8306 |
5.6914 |
6.0652 |
| CoII |
6.0737 |
5.1416 |
4.7736 |
5.1089 |
5.4042 |
5.1880 |
5.1880 |
5.1880 |
5.7228 |
6.0085 |
| nT |
CoI |
6.3457 |
6 |
6.5318 |
7.0326 |
6.4293 |
6.3614 |
6.3457 |
6 |
6.3457 |
7.0326 |
| CoII |
6.5318 |
6 |
6.2579 |
6.0544 |
6 |
6.4293 |
6.4293 |
6.4293 |
6.3614 |
6.5043 |
| nl |
CoI |
0.6543 |
1.2558 |
0.4683 |
0.9674 |
1.1413 |
0.6386 |
0.6543 |
1.1494 |
0.6543 |
0.9674 |
| CoII |
0.4683 |
0.8584 |
1.4843 |
0.9456 |
0.5958 |
1.1413 |
1.1413 |
1.1413 |
0.6386 |
0.4958 |
| m3d |
CoI |
1.9628 |
1.8837 |
1.4872 |
1.9674 |
1.7120 |
1.9157 |
1.9628 |
1.7241 |
1.9628 |
1.9674 |
| CoII |
1.4872 |
1.2876 |
0.7421 |
0.9456 |
0.8937 |
0.5707 |
1.7120 |
1.7120 |
1.9157 |
1.4047 |
| R(l)/Å |
R |
1.4721 |
1.5174 |
1.5093 |
1.5353 |
1.4932 |
1.5112 |
1.4600 |
1.4530 |
1.4790 |
1.4629 |
| CoI |
1.1816 |
1.1938 |
1.1692 |
1.1444 |
1.1331 |
1.1806 |
1.1816 |
1.1923 |
1.1816 |
1.1444 |
| CoII |
1.1692 |
1.1880 |
1.1617 |
1.2020 |
1.1842 |
1.1331 |
1.1331 |
1.1331 |
1.1806 |
1.1710 |
| Table 3. Analyses of valence electronic structures of RCo5. In this table, nc, nl, m3d, and nT denote the numbers of the covalent electrons, the lattice electrons, the magnetic electrons, and the total number of the electrons, respectively; σ is the number of quantum states. The rare earth element has a piece of the hybridization table, which possesses 10 kinds of quantum states. Element Co has 8 pieces of the hybridization table (A1, A2, B, C1, C2, D, E1, E2), and each of them records 18 kinds of quantum states. . |
4. Calculated results and discussion4.1. Crystal structures of RCo5 compoundsRCo5 compounds crystallize into the hexagonal CaCu5-type structure with a space group of P6/mmm, and the atomic occupations are 1a for R, 2c for CoI, 3g for CoII, respectively. The lattice parameters of RCo5 compounds and the valence bond lengths of R–Co and Co–Co bonds are listed in Table 1. Based on EET, the shortest atomic bond will seriously hamper the magnetization direction, whose density of covalent electron pairs (nα) is the largest. In Table 1, the valence bond length of CoI–CoII is the shortest, which indicates the interactions between CoI–CoII is the strongest, and R–CoI is the second strongest, respectively. As previously reported,[9] the R–R exchange interaction, which is much weaker than that of R–Co, could be ignored in this calculation. The atomic radii of rare earth elements except for Y element decrease gradually with increasing the atomic number of rare earth element,[27] which causes the lattice parameters and the valence bond lengths to decrease. The shorter the valence bond length is, the stronger the cohesive energy between the bonded atoms is. As for the abnormal decreases of those parameters in CeCo5 compound, the cohesive energy might be affected by the mixed valence of Ce-ion (+3, +4).
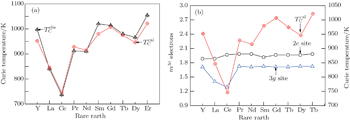
4.2. Calculation of magnetic moment and Curie temperatureThe calculated results of magnetic properties are given in Table 2. As shown in Figs. 1 and 2, the calculated results are in good agreement with the experimental results. It reveals that the magnetic moment of CoI is larger than that of CoII, which is consistent with the result of neutron diffraction experiment.[28] The moment of CoII at 3g is weakened due to the exchange coupling interaction between CoII at 3g and R at 1a site. According to Fig. 1, it can be found that the moments of RCo5 are strongly related to those of rare earths. The change tendency of RCo5 (R is light rare earth) is similar to that of R. However, in the heavy rare earth regime, the two moment curves seem to be axisymmetric due to the negative moment of the heavy rare earth. The change tendencies of Curie temperature of the RCo5 compounds are related to those of two kinds of Co atoms as shown in Fig. 2, for the heavy rare earth element (HRE) -based RCo5 are ordered ferrimagnetically. In other words, the 3d magnetic electrons m3d of LRE in LRECo5 and YCo5 compounds exhibit parallel alignment to those of Co while antiparallel in HRECo5 compounds. The direction of the arranged magnetic electrons in Co atoms is defined as being positive, and that in R atoms is positive in LRECo5 compounds and it is negative in HRECo5 compounds. For the HRECo5 compounds, the 4f electrons contribute to the moment, the molecular moment equals the sum of 3d and 4f moments, which are negative in the calculated results as presented in Eq. (7). It is nicely consistent with the observed result as shown in the inset of Fig. 1. Figure 2 shows the relationships between  and
and  as well as
as well as 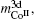 where it can be seen that the change tendency of
where it can be seen that the change tendency of  curve seems to be similar to that of
curve seems to be similar to that of  curve. It implies that the exchange coupling between CoI and R affects the Curie temperature of RCo5. For Co atoms, the moment of CoI is more than that of CoII atom. In general, Curie temperatures of RCo5 are proportional to the average moments per bond number, which reveals the anisotropies of the magnetic exchanging interactions along various bonds. Except for the contribution of 3d–3d or 3d–4f interaction to the ferromagnetic order, the lattice electrons also have an influence on the Curie temperature. As for SmCo5, the large error between the calculated and observed results for Curie temperature might be caused by the electronic transformations from lattice electrons nl into 3d magnetic electrons
curve. It implies that the exchange coupling between CoI and R affects the Curie temperature of RCo5. For Co atoms, the moment of CoI is more than that of CoII atom. In general, Curie temperatures of RCo5 are proportional to the average moments per bond number, which reveals the anisotropies of the magnetic exchanging interactions along various bonds. Except for the contribution of 3d–3d or 3d–4f interaction to the ferromagnetic order, the lattice electrons also have an influence on the Curie temperature. As for SmCo5, the large error between the calculated and observed results for Curie temperature might be caused by the electronic transformations from lattice electrons nl into 3d magnetic electrons  at 3g site (see Fig. 3(c)).
at 3g site (see Fig. 3(c)).
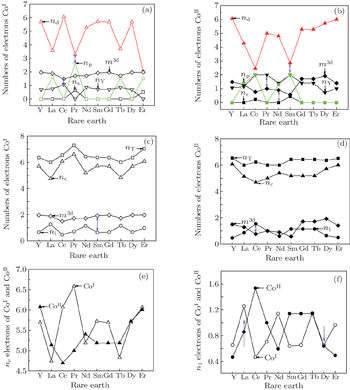
4.3. Analysis of correlation between electronic structure and magnetic propertyThe calculated valence electronic structures are given in Table 3, and the corresponding electronic transformations are shown in Fig. 3. According to the electron function in electronic binding, the electrons are classified as four categories by EET, which are magnetic electrons, dump pair electrons, lattice electrons, and covalence electrons, denoted by m3d, nY, nl, and nc respectively. The so-called dump pair electrons represent either a bonding or an anti-bonding electron with their resulting bonding power mutually canceled by each other. The lattice electron, nl is empirically introduced as a physical quantity, which comes from s electrons. According to the modern quantum theory, the s electrons might reach an energy level above the Fermi energy if the s energy bandgap is wide. In this case, the electrons can hover in lattice space, therefore the electron is so-called lattice electron. Here, two of the four categories of electrons we are talking about, nl and nc, take part in the formation of an atomic bond by electronic transformation. The nT equals the sum of nl and nc.
Figures 3(a) and 3(b) show the electronic transformations among s, p, d, nY, and m3d electrons in CoI and CoII atoms, respectively. For either of CoI or CoII, the electronic transformation between p and d electrons happens in the form of equivalent electrons. As for the electronic transformation between the m3d and nY electrons, it reveals the correlation between m3d and d electrons. When the m3d meets with the d electrons, with their resulting bonding power mutually canceled by each other and their spins are opposite to each other, the electron transformation between m3d and nY occurs. The number variation of m3d depends upon the electrons transforming from the other electrons into the 3d magnetic electrons. The final numbers of m3d electrons in CoI and CoII atoms make their differences of the contributions to molecular moments and Curie temperature. For Co atoms, the moment of CoI is bigger than that of CoII, which is due to the decrease of m3d electrons induced by electron transformation between m3d and nc of CoI. Figures 3(c) and 3(d) show the electronic transformations between nl or nc or nT and m3d electrons in CoI- and CoII atoms, respectively. The exchange coupling interactions between m3d and nl of CoII in LRECo5 compounds are the strongest, while those of CoI- in HRCo5 compounds are the strongest. Taking CeCo5 for example, the numbers of nl in CoI- is the least and the numbers of nl in CoII is the largest, which keep the balance in the final number of nl. The nl originates from the s orbital electrons, and s mainly derives from the equivalent p and d electrons. For the HRECo5 compounds, the 4f electrons also contribute to moments, the molecular moment equals the sum of 3d and 4f moments. Figures 3(e) and 3(f) show that the electronic numbers of nl and nc electrons at CoI and CoII crystal positions change with the rare earth elements. The changes seem to be the same.
5. Conclusions and perspectivesThe empirical electron theory of solids and molecules (EET), which is based on the Pauling’s valance theory, is used to investigate the valence electronic structures and multiple physical properties of solid state materials. The theoretical model is simpler with fewer theoretical parameters than the first principle and the molecular field theory. In this study, the magnetic moments and Curie temperatures of RCo5 (R = rare earth) intermetallics are systematically studied with EET.
The calculated results reveal that the moments of RCo5 intermetallics originate mainly from the 3d electrons of Co atoms and 4f electrons of rare earth, the s electrons also affect the magnetic moments by the hybridization of d and s electrons. The contribution to the moment depends on the sublattices of Co and R atoms, and it is found that the moment of Co atom at 2c site is bigger than that at 3g site due to the bonding effect between R and Co associated with a electron transformation from 3d electrons into covalence electrons. In the heavy rare-earth-based RCo5 intermetallics, the contribution to the molecular moment originates from 3d and 4f electrons. Curie temperatures of RCo5 are proportional to the average moment along the various bonds, and it indicates the magnetic anisotropy along the various crystal directions. The covalence electrons and lattice electrons also affect the Curie temperature. RCo5 intermetallic compounds show strong relationships between their valence electronic structure and magnetic properties. Except for the 3d–3d or 3d–4f exchange coupling interaction contributing to the ferromagnetic order, the lattice electrons also affect the Curie temperature.