† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51501017).
The structural, electronic, and magnetic properties of double perovskite Sr2FeReO6 containing eight different imperfections of FeRe or ReFe antisites, Fe1–Re1 or Fe1–Re4 interchanges, VFe, VRe, VO or VSr vacancies have been studied by using the first-principles projector augmented wave (PAW) within generalized gradient approximation as well as taking into account the on-site Coulomb repulsive interaction (GGA+U). No obvious structural changes are observed for the imperfect Sr2FeReO6 containing FeRe or ReFe antisites, Fe1–Re1 or Fe1–Re4 interchanges, or VSr vacancy defects. However, the six (eight) nearest oxygen neighbors of the vacancy move away from (close to) VFe or VRe (VO) vacancies. The half-metallic (HM) character is maintained for the imperfect Sr2FeReO6 containing FeRe or ReFe antisites, Fe1–Re4 interchange, VFe, VO or VSr vacancies, while it vanishes when the Fe1–Re1 interchange or VRe vacancy is presented. So the Fe1–Re1 interchange and the VRe vacancy defects should be avoided to preserve the HM character of Sr2FeReO6 and thus usage in spintronic devices. In the FeRe or ReFe antisites, Fe1–Re1 or Fe1–Re4 interchanges cases, the spin moments of the Fe (Re) cations situated on Re (Fe) antisites are in an antiferromagnetic coupling with those of the Fe (Re) cations on the regular sites. In the VFe, VRe, VO, or VSr vacancies cases, a ferromagnetic coupling is obtained within each cation sublattice, while the two cation sublattices are coupled antiferromagnetically. The total magnetic moments μtot (μB/f.u.) of the imperfect Sr2FeReO6 containing eight different defects decrease in the sequence of VSr vacancy (3.50), VRe vacancy (3.43), FeRe antisite (2.74), VO vacancy (2.64), VFe vacancy (2.51), ReFe antisite (2.29), Fe1–Re4 interchange (1.96), Fe1–Re1 interchange (1.87), and the mechanisms of the saturation magnetization reduction have been analyzed.
Over the past few decades, magnetic double perovskites with a general chemical formula of A2 BB′O6, where A is an alkaline earth metal or rare earth metal cation, and B and B′ are 3d and 4d/5d transition-metal (TM) cations, respectively, have been studied, especially the finding of the intrinsic tunneling magnetoresistance (TMR) effect at room temperature in Sr2FeMoO6[1] and Sr2FeReO6[2] revives the extensive study of the double perovskites A2 BB′O6. Bandstructure calculations reveal that they have a ferrimagnetic (FiM) half-metallic (HM) character with completely (100%) spin-polarized transport properties at the Fermi level.[1,2] The Curie temperatures TC of Sr2FeMoO6 and Sr2FeReO6 are found to be fairly high, as high as 415 K and 401 K, respectively, making them potential candidates for industrial applications in magnetoresistive[3] and spintronics[4] devices at room temperature. Besides experimental works,[1,2,5–13] various first-principles methods[1,2,14–19] including the local spin density approximation (LSDA) and the generalized gradient approximation (GGA) have been used to investigate the electronic and magnetic properties of the ordered Sr2FeMoO6 and Sr2FeReO6. It has been found that at the ground state, the Fe3+(3d5) is in the high spin state of S = 5/2 according to Hund’s rule, and Mo5+(4d1) and Re5+(5d2) are highly ionized with valence spin states of S = 1/2 and S = 1, respectively. Each of the two TM sites, namely, the Fe3+ (3d5, S = 5/2) and Mo5+ (4d1, S = 1/2) or Re5+ (5d2, S = 1) sites, is believed to be ferromagnetically (FM) arranged within each sublattice, while the two sublattices are coupled antiferromagnetically (AFM), giving rise to the total spin magnetic moments of 4 μB and 3 μB per formula unit (f.u.) for Sr2FeMoO6 and Sr2FeReO6, respectively. The smaller saturation magnetizations of 3 μB/f.u. and 2.7 μB/f.u. at 4.2 K for Sr2FeMoO6[1] and Sr2FeReO6[2] respectively are attributed to the mis-site-type disorder of the TM sites.[20–22]
Growth of the double perovskites Sr2FeMoO6 and Sr2FeReO6 would always lead to a certain degree of imperfections. For instance, Kobayashi et al.[1] found that 13% of the Fe and Mo cations in their sintered Sr2FeMoO6 are not on the respective sublattices. It is theoretically and experimentally shown that the ordering of the TM Fe and Mo/Re as well as the oxygen vacancy are the key factors determining the macroscopic magnetic properties of Sr2FeMoO6 and Sr2FeReO6. The antisite defects, which are defined as the misplacement of Fe in Mo/Re positions and vice versa, and the oxygen vacancy generally decrease the saturation magnetization and the Curie temperature TC.[1,9,10,20–32] Among those works, only Retuerto et al.[32] investigated the effects of the inherent imperfections on the properties of Sr2FeReO6 experimentally, they found that the sample prepared by a soft-chemistry technique presents 75% of Fe/Re cationic order and an enhanced Curie temperature of 445 K, while the one prepared by the high-pressure method generates a complete Fe/Re cationic order and a superior saturation magnetization. The other experimental and theoretical works on Sr2FeReO6 are about the external impurity substitution defects. Experimentally, Jung et al.[9] found that the FiM behavior of Sr2FeReO6 changes to the AFM upon Sb substitution. Jung et al.[10] studied the effects of diamagnetic dilution of the Fe sublattice on the structural and magnetic properties of Sr2Fe1−xGaxReO6. They found that the amount of antisite disorder increases with increasing Ga content. For 0 < x < 0.4, only a tetragonal and magnetically ordered phase is detected. A phase separation into a tetragonal phase and a cubic phase is detected for x ≥ 0.4. The soft-chemistry method was also used by Blanco et al.[33] to fabricate Sr2FeReO6, Sr2FeRe0.9Nb0.1O6, and Sr2FeRe0.9Ta0.1O6. The lower magnetic moments compared to the theoretical ones were attributed to the antisite disorder of 25% in the B and B′ positions. Blasco et al.[34] found that the replacement of Sr by La leads to an increase of the unit cell volume and a rise of the Curie temperature. However, both the saturated magnetic moment and the room-temperature magnetoresistance decrease with increasing La content. Theoretically, the effects of Cr substituting for Fe,[35] La substituting for Sr,[36] and Tc substituting for Re[37] on the structural, electronic, and magnetic properties of Sr2FeReO6 have been investigated by using the first-principles calculations within the density function theory (DFT) framework. Therefore, in this paper, on the point of view of the HM character and magnetization reduction, the structural, electronic, and magnetic properties of the imperfect double perovskite Sr2FeReO6 containing eight different inherent defects of Fe antisite (FeRe), Re antisite (ReFe), Fe1–Re1 interchange (Fe1–Re1), Fe1–Re4 interchange (Fe1–Re4), Fe vacancy (VFe), Re vacancy (VRe), O vacancy (VO), and Sr vacancy (VSr) are studied by using first-principles projector augmented wave (PAW) within generalized gradient approximation as well as taking into account the on-site Coulomb repulsive interaction (GGA+U).
The calculations are performed using the Vienna ab initio simulation package (VASP) based on the DFT.[38–41] The interaction between electron and ionic core is represented by the projector augmented wave (PAW) potentials,[42] which are more accurate than the ultra-soft pseudopotentials. To treat the electron exchange and correlation, we choose the Perdew–Burke–Ernzerhof (PBE)[43] formulation of the generalized gradient approximation (GGA). We take into account the on-site Coulomb repulsive interaction (GGA+U) with the effective U parameters of 2.0 eV for Fe and 1.0 eV for Re,[15,44] which yields the correct ground state structure of the strong correlation system. A conjugate-gradient algorithm is used to relax the ions into their ground states, and the energies and the forces on each ion are converged within 1.0 × 10−4 eV/atom and 0.02 eV/Å, respectively. The cutoff energy for the plane-waves is chosen to be 450 eV. The Sr 4s24p65s2, Fe 3d64s2, Re 5d56s2, and O 2s22p4 electrons are treated as valence electrons. The k-points are sampled according to the Monkhorst–Pack automatic generation scheme with their origin at the Γ point[45] and 10 divisions in each direction, together with a Gaussian smearing broadening of 0.1 eV.
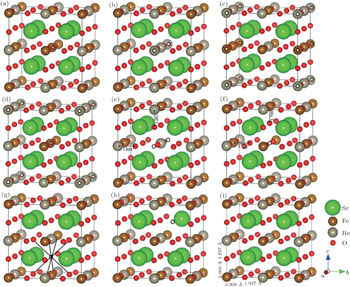
In order to compare the electronic structures of the imperfect and the perfect Sr2FeReO6 on the same footing, the calculations are all performed by constructing a supercell of 4 f.u. (Sr8Fe4Re4O24). As compared with Fig.
The relaxed structures of 4 f.u. Sr2FeReO6 with eight different defects (marked with circles) are shown in Figs.
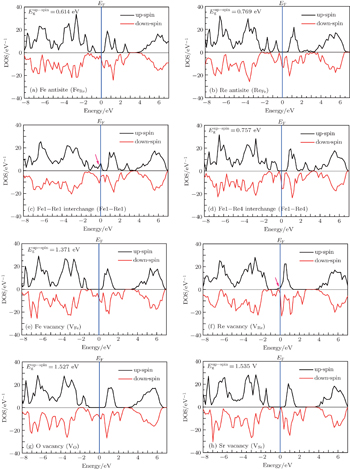
In order to investigate the effects of the defects on the HM character of the double perovskite Sr2FeReO6, which are very important for its application in spintronic devices, we show in Fig. 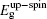
Now we turn to examine the disappearing reason of the HM character of the imperfect Sr2FeReO6 containing Fe1–Re1 interchange defect (see Figs. 
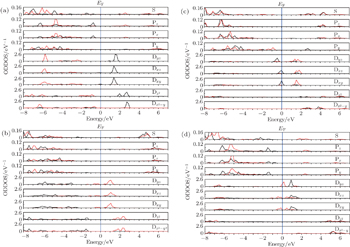
In order to investigate the effects of the defects on the spin coupling and magnetic moments, we show the spin or difference charge densities (ρspin = ρup-spin − ρdown-spin) in Fig.
The initial fractional coordinates (x, y, z) and local magnetic moments μ (μB) of the Fe and Re atoms on the regular site and antisites, the total magnetic moments μtot (μB/f.u.), the HM characters, and the up-spin band gaps 
| Table 1. The initial fractional coordinates (x, y, z) and local magnetic moments μ (μB) of the Fe and Re atoms on the regular site and antisites, the total magnetic moments μtot (μB/f.u.), half-metallic characters, and up-spin band gaps |
From the aspects of HM character and magnetization reduction, the structural, electronic, and magnetic properties of the imperfect Sr2FeReO6 containing eight different defects of FeRe or ReFe antisites, Fe1–Re1 or Fe1–Re4 interchanges, VFe, VRe, VO, or VSr vacancies have been studied by using the first-principles projector augmented wave (PAW) potential within the generalized gradient approximation as well as taking into account on-site the Coulomb repulsive interaction (GGA+U). The following results have been obtained.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 |