† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 61504128, 61504129, 61274041, and 11275228), the National Basic Research Program of China (Grant No. 2012CB619305), the National High Technology Research and Development Program of China (Grant Nos. 2014AA032603, 2014AA032609, and 2015AA010801), and the Guangdong Provincial Scientific and Technologic Planning Program, China (Grant No. 2014B010119002).
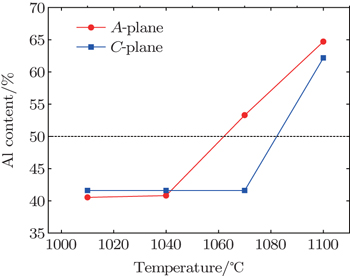
The aluminum incorporation efficiencies in nonpolar A-plane and polar C-plane AlGaN films grown by metalorganic vapour phase epitaxy (MOVPE) are investigated. It is found that the aluminum content in A-plane AlGaN film is obviously higher than that in the C-plane sample when the growth temperature is above 1070 °C. The high aluminum incorporation efficiency is beneficial to fabricating deep ultraviolet optoelectronic devices. Moreover, the influences of the gas inlet ratio, the V/III ratio, and the chamber pressure on the aluminum content are studied. The results are important for growing the AlGaN films, especially nonpolar AlGaN epilayers.
AlGaN alloys are important for optoelectronic devices such as light emitting diodes (LEDs),[1–3] laser diodes (LDs)[4] and photodetectors (PDs)[5] in the UV spectral region from 200 nm to 365 nm, which can be tuned by changing the Al content in the AlGaN ternary alloy. These devices can be applied to important areas such as disinfection/sterilization,[2] homeland security,[2] and secure space-to-space communication.[6] However, the quantum efficiencies of these devices are still poor due to the high defect densities, high contact resistances, and low light extraction efficiencies, etc.[1,2] Moreover, the device fabricated on traditional C-plane AlGaN suffers the large quantum confined Stark effect (QCSE), which is caused by the strong polarization effect in the [0001] growth direction.[7] The QCSE would lead to the spatial separation of electron and hole wave functions, reducing the optical transition probability and the device efficiency.[7] Therefore, without a polarization field in the growth direction, the AlGaN optoelectronic device may achieve a high device efficiency.
As is well known, high Al content AlGaN film for a deep UV device is difficult to grow due to the low Al incorporation efficiency in AlGaN. A lot of work has been done on studying the mechanism of governing the Al content in the as-grown C-plane AlGaN and it has been found that many growth parameters are relevant.[8–10] However, there are few reports on the growth condition for the nonpolar AlGaN film, especially A-plane AlGaN.
In this paper, a comparison of the Al content between nonpolar A-plane and polar C-plane AlGaN films grown by MOCVD is made. The influences of the growth parameters, such as the trimethylaluminum (TMAl) and ammonia flow rates, the reactor pressure, and the growth temperature on the aluminum content are investigated.
The AlGaN samples were grown in a homemade metalorganic vapour phase epitaxy (MOVPE), which has been described in our previous paper.[11] The A- and C-plane AlGaN films were grown on the ∼ 1 μm A- and C-plane GaN templates, respectively. The methods of fabricating high-quality A- and C-plane GaN templates can be found elsewhere.[12,13] In all the experiments, the total flow rate of TMAl and trimethylgallium (TMGa) was kept constant (50 μmol/min), while the flow rate ratio between TMAl and (TMAl + TMGa) was varied from 5% to 75%. Hydrogen was used as the carrier gas. The V/III ratio which is the flow ratio between elements of groups V and III, was changed from 900 to 3600 by adjusting the ammonia flow rate. When the ammonia flow rate was changed, the hydrogen flow rate was also adjusted in order to maintain a constant total flow rate of the inlet gas. The chamber pressure was varied between 30 Torr to 70 Torr (1 Torr = 1.33322×102 Pa), while the growth temperature was in a range of 1010 °C–1100 °C. The growth time was fixed at 15 min. The compositions of the AlGaN epilayer are measured by the x-ray diffractometry (XRD: Philips X’ Pert Pro diffractometer).
Figure
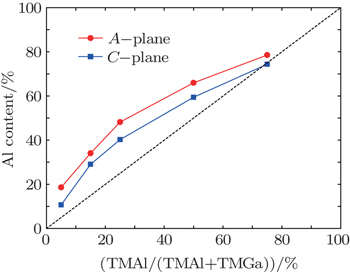 | Fig. 1. Plots of Al content versus TMAl gas inlet ratio for A- and C-plane AlGaN films, where the dashed line corresponds to the mass-transport limit. |
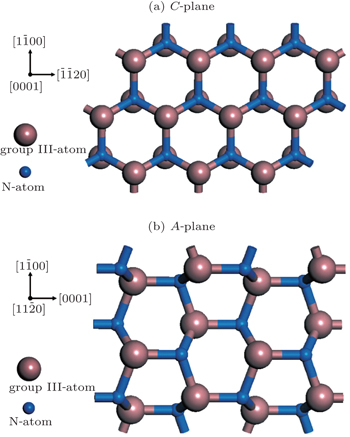
Moreover, the Al content of the A-plane AlGaN sample is higher than that of the C-plane one, which might be due to the different atomic bond configurations on C- and A-planes. At a fixed temperature, the Ga desorption rate depends on the number of surface nitrogen atom dangling bonds and the density of nitrogen atoms. As shown in Fig.
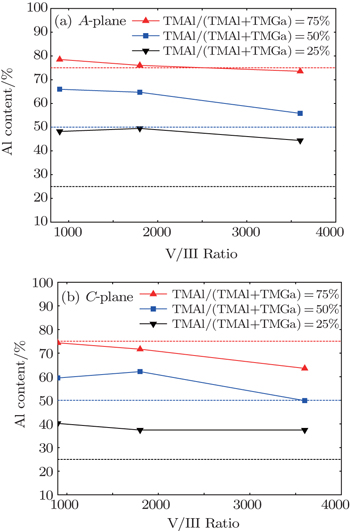
The dependencies of Al content values in A- and C-plane AlGaN on chamber pressure are shown in Fig.
When the V/III ratio increases, the NH3 partial pressure increases and the H2 partial pressure decreases since the total flow rate is kept unchanged. The increased NH3 partial pressure will enhance the parasitic reaction between TMAl and NH3, thus reducing the Al incorporation efficiency. Meanwhile, the reduced H2 partial pressure reduces the Ga desorption rate,[16,17] which also is harmful for Al incorporation in AlGaN. Both of the above two factors result in the reduction of Al content in AlGaN as the V/III ratio increases as shown in Fig.
In previous studies, it was found that the parasitic reactions between TMAl and NH3 increase at an elevated growth temperature,[6] which is not beneficial to increasing the Al content in AlGaN film. On the contrary, the desorption of Ga atoms becomes more rapid when the temperature is increased, which is beneficial to increasing that. These two factors make the relation between the Al content and the growth temperature unclear. In Fig.
In this paper, we carry out the experimental study of the Al content values in nonpolar A-plane and polar C-plane AlGaN films grown by MOVPE. The deviation of Al content from the transport limit is mainly due to the parasitic reaction and the Ga atom desorption. Low chamber pressure, low V/III ratio, and high growth temperature are beneficial to the Al incorporation. Moreover, it is found that the Al content in the A-plane AlGaN sample is larger than that in the C-plane sample when the growth temperature is above 1070 °C, which might be due to the difference in atomic bond configuration between on C-plane and on A-plane. Our results indicate that the A-plane AlGaN films are more suitable for fabricating deep-UV optoelectronic devices, in which a high Al content AlGaN is necessary.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 |