† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 50974016 and 50071014).
Powder metallurgy (PM) superalloys are an important class of high temperature structural materials, key to the rotating components of aero engines. In the purview of the present challenges associated with PM superalloys, two novel approaches namely, powder preparation and the innovative spray-forming technique (for making turbine disk) are proposed and studied. Subsequently, advanced technologies like electrode-induction-melting gas atomization (EIGA), and spark-plasma discharge spheroidization (SPDS) are introduced, for ceramic-free superalloy powders. Presently, new processing routes are sought after for preparing finer and cleaner raw powders for disk superalloys. The progress of research in spray-formed PM superalloys is first summarized in detail. The spray-formed superalloy disks specifically exhibit excellent mechanical properties. This paper reviews the recent progress in innovative technologies for PM superalloys, with an emphasis on new ideas and approaches, central to the innovation driving techniques like powder processing and spray forming.
Presently, powder metallurgy (PM) superalloys have become the prime choice as high-performance materials for aero-engine turbine disks. However, PM superalloys suffer microstructural limitations in the form of powder-particle boundaries (PPB), thermally introduced porosity (TIP), inclusions and other defects, introduced by the its rather unique processing requirements.[1–5] As a state of the art, the problems of PPB and TIP have been significantly improved, however the occurrence of inclusions still remains a challenge.[6] The mechanical properties of the PM superalloys, especially the low cycle fatigue life (LCF), can be considerably affected by the inclusions.[7–9] The sizes of starting powders, in large parts determine the sizes of the inclusions. Therefore, reducing the initial powder size is key to the control of inclusions. Hence, preparation of fine and impurity free powders is an effective approach to solving the problems related to inclusions.[10]
Raw powders are the basis and prerequisite for the preparation of the turbine-disks out of PM superalloys. At present, the technologies for powder preparation mainly include argon atomization (AA) method, and plasma rotating electrode processing (PREP). AA is widely used in American and European countries for preparing the superalloy powders. In AA technology, refractory components such as smelting crucible and nozzle stay in close proximity to the active material, as a result a small proportion of non-metallic inclusions are introduced into the prepared powders.[11–13] On the other hand, PREP is widely used in Russia for preparing the superalloy powders. However, as part of an inherent limitation of PREP, it is difficult to obtain finer powders, since the rotational speed of the electrode rod sets a lower limit to the particle size. Also, a small quantity of coarse residual inclusions are commonly found in PREP powders.[14,15] To manoeuvre further reduction in the occurrence of parasitic inclusions, researches on new preparation technologies for ultra-purity fine powders are essential. This is what determines the persisting trend in the development of PM superalloys.[16,17]
In addition, out of economic considerations, it has become important to reduce the cost of manufacturing of aero-engine turbine disks.[18] The combination of low cost and high performance is another trend associated with the development of disk superalloy.[19] The low cost manufacturing of the disc shaped components is increasingly emphasized and valued. Recently, a Cast & Wrought processing route to the preparation of disk superalloy with less than 40% volume fraction of the gamma prime phase has been reported.[20] The reported method could effectively save manufacturing costs better in comparison with the traditional PM route. However, it should be noted that an exceeding gamma prime phase volume fraction (> 40%) could lead to difficulties in forming, especially in forging of billets. Therefore, it is necessary to further optimize the low cost manufacturing of PM disk superalloy.
As a part of the research advancement towards optimized superalloy powder preparation technologies, novel methods, namely electrode induction melting gas atomization (EIGA), and the spark plasma discharge spheroidization (SPDS) were proposed. EIGA has been used in the preparation of alloys based on TiAl,[21] Ti,[22] Zr,[23] and Mg systems.[24] Materials and Electrochemical Research (MER) Corp. of Tucson (Arizona, USA) has reported the use of a method similar to SPDS, for producing ultra-fine metal powders.[25] However, the preparation of superalloy powders by means of EIGA and SPDS routes have not been reported anywhere. This paper also presents a preliminary contribution to the developments of these methods.
In our study of low cost manufacturing of turbine disk, we have utilized spray-forming technology to prepare an ingot of PM disk superalloy. Spray forming is an advanced technology of rapid-solidification, which stems from metal atomization and powder metallurgy. It possesses great potential for lowering the costs and shortening the manufacturing process of the superalloy based turbine disk-blanks, by means of the Cast & Wrought and the PM route.[26] In recent years, most of the researches focusing on spray-forming, in conjunction with Cast & Wrought superalloys, have been already put into practice.[27–35] However, the work related to spray-forming of PM superalloys still lacks a rigorous and systematic approach.[36] Therefore, it becomes indispensable to further study spray-formed PM superalloys.
In this paper, a thorough research on the technology for preparation of superalloy powders using EIGA and SPDS, as well as the low cost manufacturing of PM disk superalloys via spray-forming route is presented. Current progress of research in the associated technologies is also reported. The aim of this paper is to provide theoretical support for the development of new technologies of preparing PM disk superalloys.
The equipment of EIGA processing is shown in Fig.
EIGA technology is first used to prepare powders of disk superalloy FGH4095, a 1st generation PM superalloy in China. We have carried out research work on the equipment and technology for preparing superalloy powders, based on the working principles of EIGA. The first ever EIGA device in China, as an independent innovation, has been fabricated. The work in the direction of preparation processing and control mechanism for highly pure powders is done by optimizing the power parameters and nozzle design.
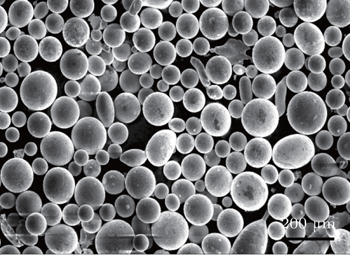
The morphology of superalloy FGH4095 powders prepared by the EIGA technology is shown in Fig.
The solidification microstructure of the surface of relatively small particles (20 μm to 50 μm) exhibits a cellular structure. Dendritic microstructure starts to appear for the particles in a range from 50 μm to 80 μm in diameter, however the proportion of the cellular structure remains larger. The dendritic surface solidification increases in the particles with sizes of about 80 μm to 100 μm, accompanied by a decrease in the cellular morphology. The solidification microstructure of particles with more than 100 μm of size is purely dendritic. The trend, where the solidification microstructure changes from cellular to dendritic shape with increasing particle size, is observed with higher consistency. Figure
 | Fig. 4. Solidification microstructures of (a) surface and (b) interior of EIGA powder particle respectively. |
The bulk solidification microstructure of FGH4095 powders mainly includes cellular structure, developed cellular structure, and dendrites. Smaller particles (< 50 μm) exhibit developed cellular structures, whereas intermediate particles (50 μm to 80 μm) are largely found to have cellular type structure. Likewise, larger particles (> 100 μm) are found to be composed of dentrites, a modicum of them even manifest developed secondary dendrites (Fig.
After careful observations, no hollow particles are found for sizes less than 150 μm. Very few hollow particles are observable in the larger particles (> 150 μm). The hollow particles are one of the main sources of thermally introduced porosity (TIP) in PM superalloys. It can be said that PM superalloy products made from the EIGA powders basically eliminate hollow particle contribution to TIP, so that the performance can be greatly improved.
Current literature shows that the types of inclusions in AA and PREP powders can be divided into following three kinds: (i) ceramic and slag inclusions, which mainly come out of master alloy and the process of powder preparation, (ii) organic inclusions, which mainly come out of powder treatment and transportation process, (iii) dissimilar metal inclusions, which mainly come out of high melting point segregations in the master alloy.[7–10,38] For comparison, the main chemical compositions of the inclusions in AA, PREP, and EIGA powders are listed together in Table
| Table 1. Main elements of inclusions in AA, PREP, and EIGA powders. . |
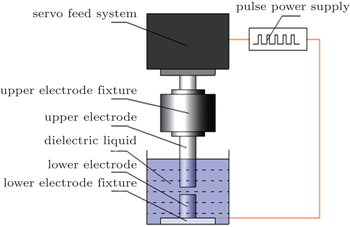
Primarily, the SPDS process takes place during the spark discharge between pre-alloyed charges immersed in a dielectric fluid. After a complex plasma creation and breakdown, the molten and subsequently evaporated material is ejected from the electrodes and quenched in the dielectric medium. This material ultimately condenses as powder in the dielectric medium. Since the powders are quenched in dielectric, they undergo extremely rapid cooling. Compared with the well-known atomization process such as argon atomization (AA) and plasma rotating electrode process (PREP), this novel process can produce superfine powders at a much faster cooling rate (∼ 107 K/s).
The main steps of SPDS include the dielectric breakdown and the formation of discharge channel followed by the energy distribution and heat transmission, the rapid solidification, and the ejection and transfer of product. Figure
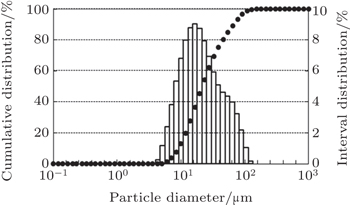
PM Superalloy FGH4096, a 2nd generation PM superalloy in China, is chosen as the test material. Fine spherical particles with sizes less than 40 μm are obtained via the SPDS process. The sphericities and particle sizes of the powders for the three different pilot dielectric liquids used, namely kerosene, alcohol with liquid argon, and pure alcohol are different. FGH4096 powders show higher sphericity and finer particle sizes for alcohol/liquid argon used as the dielectric medium (Fig.
 | Fig. 6. Morphology images of the SPDS powders prepared in alcohol with liquid argon (a) and fine powders obtained by optimizing the processing (b). |
The solidification microstructure at the surface of the SPDS powder shows no dendritic and cellular morphologies, while its bulk manifests a spherical solidification structure; in effect the overall microstructure of the powder is more uniform. Main causes of such a microstructure are that the particle sizes are much smaller than those obtained by the traditional processes (AA and PREP), and that the solidification rate (cooling speed) of the powder is also relatively high (1 order of magnitude higher). Therefore, it is not easy to form dendrites; rather the powder may assume to have a spherical structure.
The research work relating to inclusions in SPDS powder are done. The SPDS powder products have a broad potential market. Besides the preparation of PM superalloys for making turbine disks, the method can also be used for injection molding and rapid laser prototyping technology.
In short, the superalloy powders prepared by EIGA and SPDS processing have the advantages in high sphericity, small particle size, uniform microstructure and less inclusion contents. A new way of preparing disk superalloy powders is hence opened up by these methods.
Superalloy powders often contain difficultly in sintering elements like Cr, Ti, and A1. These elements are easy to oxidate at the sintering temperature, which causes the superalloy powders not to be consolidated by using the usual sintering process. For superalloy powders, it is often required to form billet under high temperature and high pressure. The main consolidation methods of superalloy powders include vacuum hot pressing, hot isostatic pressing, spark sintering, hot extrusion, forging, etc. At present, hot isostatic pressing (HIP) is used commonly for consolidating AA, PREP, EIGA, and SPDS powders. HIPed billets are isothermally forged to produce turbine disk in American and European countries. Whereas, as-HIP processing is adopted to manufacture disk components in Russia.[6,25] The microstructure and mechanical properties of disk superalloy are acutely affected by forming technology. Study relating to microstructure and performance of EIGA and SPDS powders are awaited to further perform.
Spray-forming is based on powder metallurgy technology. However, it avoids the associated disadvantages namely, several processing steps, high cost and contamination etc.[39] In order to reduce the manufacturing cost of the turbine disk, we have recently studied and developed the superalloys FGH4095, FGH4095M, FGH102, and others, prepared via spray-forming technology.
The master alloys are prepared by using vacuum induction melting (VIM), followed by vacuum arc remelting (VAR). The spray-forming process is set-up at University of Bremen in Germany, in conjunction with spray-forming plant SK-2. As a first step, the feedstock material is melted in a crucible by an induction coil. The crucible has a melt capacity of 20 L. The crucible and the induction coil are then placed in a vacuum chamber followed by purging of argon. At the same time, the tundish is heated via a resistance heating system. When the melting temperature is attained, the molten metal is poured into the preheated tundish and directed through the ceramic nozzle at the bottom. Further, the molten stream is atomized by a nitrogen stream through a scanning atomizer. The droplets are accelerated and finally impinged and solidified on the rotating substrate.
Specimens out of the spray-formed PM superalloy are taken from the top, middle, and bottom parts of the deposited billet. Archimedes method is used to measure the density of the sample. It is easy to compute the relative density of the specimens in different parts of the billet by using the theoretical density of the alloy, which can reflect the densification states of the different parts of deposited billet.[40] The measurement results of the spray-formed superalloy FGH102, a new 3rd generation PM superalloy in China, are reported in Table
| Table 2. Densities and relative densities of deposited billets. . |
Table
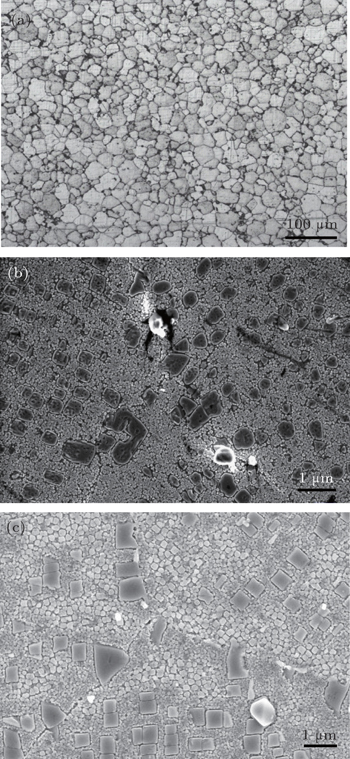
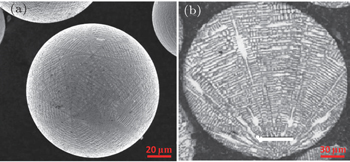
The deposited billet of the superalloys exhibits a fine structured and uniform distribution of grains. The grain structure of FGH102 is shown in Fig.
All spray-deposited billets of FGH102 alloy are processed by hot isostatic pressing (HIP) so as to improve the degree of densification. The ‘HIPed’ preforms are isothermally forged (IF) to produce several pancake-shaped forgings with about 210 mm in diameter and 40 mm in height. The disk forgings are solution heat-treated at 1130 °C for 1 h with fast cooling, and aged at 850 °C for 4 h followed by 770 °C for 8 h with air cooling. The γ′ morphology of disk forgings after the heat treatment is shown in Fig.
The key mechanical properties of the turbine disk prepared by HIP+IF pressing spray-formed superalloy FGH102, after heat treatment are listed in Table
| Table 3. Key mechanical properties of the spray-formed superalloy FGH102 disk forgings. . |
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 |