† Corresponding author. E-mail:
Project supported by the National Basic Research Program of China (Grant Nos. 2011CB932604 and 2014CB932402), the National Natural Science Foundation of China (Grant Nos. 51221264, 51172242, 51525206, and U1401243), and the Key Research Program of Chinese Academy of Sciences (Grant No. KGZD-EW-T06).
The application of wavy structures in stretchable electrochemical energy storage devices is reviewed. First, the mechanical analysis of wavy structures, specific to flexible electronics, is introduced. Second, stretchable electrochemical energy storage devices with wavy structures are discussed. Finally, the present problems and challenges are reviewed, and possible directions for future research are outlined.
Flexible electronic devices have emerged as an important field of modern electronics. They are essential for many innovative applications, such as wearable electronics, soft surgical instruments, and sensitive robotic skins. To meet the growing demand for flexible electronics, flexible electrochemical energy storage devices such as flexible supercapacitors and lithium ion batteries (LIBs) are needed. [ 1 – 4 ] So far, no clear definition for flexible electrochemical energy storage devices has been accepted. Considering the trends in electronic devices, flexible electrochemical energy storage devices can be defined as devices that can deform and work within a normal elastic range. When the external loading disappears, such devices can completely recover their original state with no additional plastic deformation or electrochemical performance deterioration. [ 5 ] Besides deformability, two features of flexible LIBs are notable: (i) ultrathin and ultra-light: compared with the conventional prismatic electrochemical energy storage devices, flexible LIBs ought to be thinner to adapt and integrate into future mobile and wearable electronic devices; (ii) various shapes: in addition to simple prismatic examples, flexible electrochemical energy storage devices should be made in various shapes to meet curved surfaces such as skin and other tissues. Among these, reversible elastic flexibility is the core feature of flexible electronics.
From the viewpoint of practical applications, the typical deformation modes of flexible LIBs include bending and stretching. In today’s experience, energy flexible storage devices usually refer to bendable devices such as bendable LIBs, supercapacitors, and solar cells. [ 2 , 3 , 6 , 7 ] Another kind is stretchable devices, the production of which is more challenging and difficult. [ 8 ]
In general, novel elastic hybrid materials and structural designs are the main routes to stretchable energy storage systems. However, developing stretchable active materials for energy storage systems is extremely difficult, because an appropriate material should meet lots of technical requirements. On the other hand, a novel stretchable structural design can buffer large scale elastic deformation, which is a viable way to achieve stretchability based on the conventional brittle inorganic materials. One of the most popular and effective strategies for designing stretchable structures is wavy structures. Such designs permit large elastic deformation by releasing the strain. Figure
Recently, intense research has been conducted on stretchable energy storage devices with wavy designs, using conventional inorganic materials. [ 10 – 12 ] This is mainly because the familiar active materials in energy storage devices show good conductivity, cost little, and are amenable to fabrication by today’s industrial technology. Integrating rigid inorganic materials with soft compliant substrates will become important in the development of flexible electronic devices.
In this brief review, we summarize the application of wavy structures in stretchable electrochemical energy storage devices. First, we introduce the mechanical analysis of wavy structures in flexible electronics. Second, we focus on stretchable electrochemical energy storage devices with wavy structures. Finally, we discuss the existing problems and challenges, and possible directions for future research.
In practice, stretching-deformation is more challenging than bending-deformation mainly due to the very limited elastic strain extensibility of the conventional electrode materials (LiCoO 2 : < 0.1%, [ 13 ] graphite: 0.1∼0.2%, [ 14 ] Si: < 0.1%, [ 13 ] carbon nanotubes: < 3.0%, [ 15 , 16 ] graphene or graphene oxide membrane: 0.6%, [ 17 ] etc.), these materials cannot accommodate large scale elastic strain. Stretching leads to fairly extensive destruction in flexible electrodes due mainly to two processes during stretching: (i) the inorganic active material itself has an extremely low elastic strain, so even a little stretching can fracture the electrode overall; (ii) the active materials may lose electronic contact due to repeated deformation. [ 18 ]
To overcome these problems, we should use innovative structures to design the electrodes. We can use structural deformation to buffer deformation of active materials during stretching. One of the most popular stretchable structural designs is the wavy or wrinkled structure. Various wavy structures have found increasingly wide utilization in flexible electronics, [ 19 – 21 ] and have progressively expanded into other fields. Song et al. [ 22 ] proposed three possible wavy structures: (i) one-dimensional (1D) wavy electrode, (ii) two-dimensional (2D) checkerboard wavy structure, and (iii) 2D herringbone structure. [ 19 , 23 , 24 ] Figure
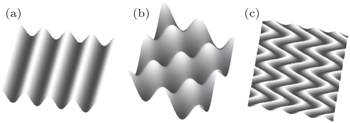 | Fig. 2. Wavy designs for flexible electronics: (a) one-dimensional wavy structure, (b) 2D checkerboard wavy structure, and (c) 2D herringbone structure. [ 22 ] |
Figure
 | Fig. 3. Fabrication process of wavy structure. [ 24 ] |
Based on the small-deformation theory, Huang et al. [ 25 ] developed a minimum energy method to investigate the buckling process under small pre-strain (0.5% < ɛ pre < 5%) for a 1D wavy structure (Fig.
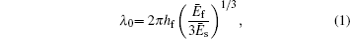
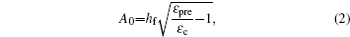

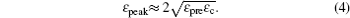
For large deformation and pre-strain (5% < ɛ pre < 30%), the wavelength increases with increasing pre-strain. Jiang et al. [ 27 ] used finite deformation theory to analyze the buckling process. Under large pre-strain, the wavelength and the amplitude can be obtained by
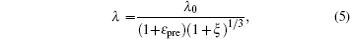
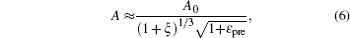

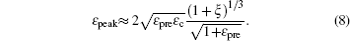
Besides 1D wavy structure, some researchers produced biaxially stretchable wavy structures on elastomeric PDMS substrate to provide full 2D stretchability. Choi et al. [ 28 ] fabricated herringbone 2D stretchable silicon films on elastic substrates. First, a controlled degree of bilateral isotropic thermal expansion was induced in an activated PDMS substrate, and silicon films were deposited on the substrate. The silicon nano membrane/PDMS structure was then cooled to room temperature to release the thermally induced pre-strain. This process led to the spontaneous formation of a 2D wavy structure. In order to investigate the 2D buckling patterns, Song et al. [ 22 ] performed an analytical study of different buckling modes, including 1D, 2D checkerboard, and herringbone structures. According to Song et al. , [ 22 ] the herringbone mode (Fig.
As stated above, electronics are mostly based on inorganic materials, such as silicon, gallium arsenide, and gallium nitride. Compatible, well developed, high performance inorganic electronic materials are a key challenge in the development of flexible electronics. Wavy structure design relies on new structural layouts based on the conventional materials and enables brittle materials to be made flexible to some extent. In accordance with the choice of materials, electrochemical energy storage devices, such as LIBs and supercapacitors have points of resemblance to ideal flexible electronics. Due to the inherently limited small strain of active materials in LIBs and supercapacitors, we can obtain only bending-deformation. Based on wavy designs for stretchable electronics, some work has been done on stretchable electrochemical energy storage devices. Table
The conventional electrochemical energy storage device consists of an electrode with a polymer separator, an aqueous or organic liquid electrolyte, a metal tab, and packaging. Preparation of electrodes for LIBs is mainly based on a slurry-casting method that requires mixing active materials with binders and conductive additives, and then casting the mixture onto a metal (Al or Cu) foil. Rigid current collectors provide the structural support and electrical conductive pathways for the electrodes. [ 3 , 40 ] As shown in Table
Suitable elastic substrate and interface properties are important to construct an effective wavy structure. Flexible substrates that are to replace rigid current collectors must meet the following requirements: (i) stability of the electrolyte: the substrate should not contribute any contaminants during electrochemical reactions and should be totally inert against the electrolyte and any other chemicals in the system; (ii) mechanical properties: the substrate should have a suitable elastic modulus and mechanical properties; (iii) surface pre-treatment of elastic substrate: we can introduce a conductive layer to enhance the electronic conductivity; surface pre-treatment also includes introducing functional groups on the surface of polymers, which can yield intact interface bonding between the active material and the substrate.
| Table 1. Stretchable electrochemical energy storage devices with 1D and 2D wavy structures. . |
Suitable substrates should have high electrolyte resistance and excellent interface properties. Due to their greater stretchability, PDMS and PET are often used as stretchable substrates. An elastomer serving as a flexible substrate inevitably contacts the electrolyte, such as liquid carbonate solvent with a high dielectric constant, H 2 SO 4 aqueous solution, etc. Yun et al. [ 41 ] measured the stability of polymeric films against electrolytes by the amount of electrolyte taken up into polymeric films. They found that the resistance of polymeric films to electrolyte absorption is in the order of: Poly (ethylene naphthalate) PEN > poly (ethylene terephthalate) PET > (polyimide) PI > poly (ether sulfone) PES. PEN is the most stable substrate in an electrolyte solution due to its high strength, superior barrier property, and high stability at elevated temperature, associated with its low expansion coefficient. In contrast, PES losts its chemical stability owing to the high diffusion of electrolyte into it.
As Table
As flexible polymers are usually insulators, the resistance of flexible substrates is much higher than that of Cu and Al foil current collectors. Therefore it is necessary to significantly lower the resistance of flexible substrates in order to obtain a performance comparable to metal foils. [ 41 ] In order to enhance the conductivity of a stretchable elastomer, a thin metal (Cu, Au, etc.) layer coated on a flexible polymer film may be favorable. Wang et al. [ 32 ] deposited gold on pre-strained SIBS substrates and released the pre-strain, obtaining buckled PPy-pTS films, as illustrated in Fig.
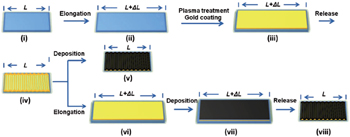 | Fig. 4. Schematic procedures for the fabrication of buckled Au and PPy-pTS films on substrates. [ 32 ] |
Another important property of the substrates is strong bonding between active materials and stretchable substrates. In order to ensure intact binding between active materials and flexible substrates, appropriate pre-treatment is important. The most widely used flexible substrate PDMS is composed of polymeric chains constructed with repeating units of –OSi(CH 3 ) 2 O–. Figure
 | Fig. 5. Illustration of the surface chemistry of PDMS and reactions occurring at the interfaces between PDMS and semiconducting nanoribbons covered with thin SiO 2 layers. [ 44 ] |
Already, wavy structures have been constructed of many dissimilar active materials, including nanocarbons (carbon nanotubes (CNTs) and graphene), polymers, and traditional LIB materials. The nanocarbons have been widely used for high energy and high power-density supercapacitors due to the materials’ unique morphologies, high surface to volume ratio, and high ionic and electronic conductivities. Moreover, their higher elastic strain enables potential applications in stretchable LIBs and supercapacitors. [ 7 , 40 , 47 , 48 ] Yu et al. [ 29 ] prepared sinusoidal SWNT macrofilms on 1D pre-stretched PDMS substrates. Figures
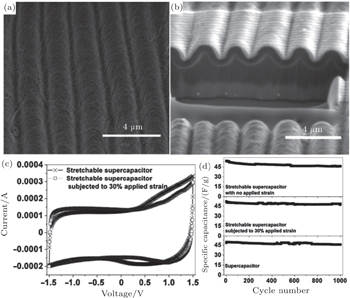 | Fig. 6. (a) and (b) SEM morphologies of buckled CNTs macrofilms on pre-strained PDMS substrates; (c) CV curves of stretchable supercapacitors with and without 30% strain; (d) specific capacitance of cycled stretchable supercapacitors with and without 30% strain. [ 29 ] |
So far, most of the research about stretchable electrochemical energy storage devices has only been about 1D wavy structures. However, biaxial pre-stretched PDMS substrates can effectively enhance the flexibility of devices by enabling them to stretch in two directions. Zang et al. [ 37 ] fabricated crumpled graphene on biaxially stretched polymer substrates. Figure
 | Fig. 7. (a) Herringbone structured crumpled graphene; (b) cycles of stretchable supercapacitors. [ 37 ] |
Another series of active materials shown in Table
In summary, strong chemical bonding, stable electrolyte substrates, and appropriate materials provide a basis for fabricating a wide range of wavy structures in stretchable devices.
Flexible electrodes with wavy structure can be significantly more stretchable, and we now know a good bit about designing and fabricating them. But lots of challenges remain to be overcome in developing stretchable electrochemical energy storage devices. (i) Although the wavy structure can improve the deformability of some materials to an extent, the resulting stretchable elastic strain is generally less than 30%–50%. (ii) So far, most wavy-structure materials are still based on the nanocarbon materials. Little research has been published on traditional LIB materials. (iii) Flexible separators, electrolytes, and tabs are needed. A flexible electrochemical energy storage device is composed mainly of flexible electrodes, separators, an electrolyte, and metal tabs. To make the overall device stretchable, all components must be compatible with the design parameters for stretching without failing in their functions.
Aiming at the above problems, the following new components should be designed and developed.
 | Fig. 8. Fabrication of noncoplanar interconnect-island structure. [ 51 ] |
Kim et al. [ 52 ] prepared a stretchable solid-state microsupercapacitor array using a serpentine wavy structure. The narrow and long serpentine metallic interconnections are encapsulated by a polyimide thin film to ensure that they are within the neutral plane (Fig.
 | Fig. 9. Wavy structure with serpentine interconnects. [ 52 ] |
Based on serpentine designs, Xu et al. [ 53 ] developed stretchable lithium batteries with a self-similar island-bridge structure. This design enables reversible stretchability up to 300%. This stretchable battery uses silicone elastomers as substrates, stretchable electrodes, and polymer electrolyte stacked sequentially. Its design is illustrated in Figs.
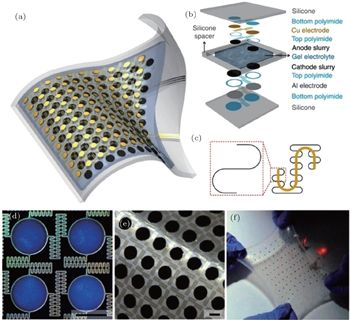 | Fig. 10. (a) and (b) Structure of stretchable LIBs; (c) and (d) stretchable current collectors with island-bridge structure; (e) island-bridge electrodes with active materials; (f) illustration of stretchable batteries. [ 53 ] |
With the development of flexible electronics, stretchable electrochemical energy storage devices have drawn intense attention. Although we have made great progress in the fabrication of flexible electrodes in recent years, obstacles still need to be overcome. Future efforts should be made as follows: (i) develop new intrinsically elastic active materials; (ii) develop new wavy structures to achieve higher flexibility, such as selfsimilar structures and serpentine structures.
Although there are still many problems to overcome, the ubiquitous potential applications of flexible LIBs with superior electrochemical and mechanical properties in stretchable electrochemical energy storage devices are greatly anticipated for the near future.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 |



