† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. U1232203, U1432104, U1332107, 11305198, and 11405199), the Program for Young Teachers Scientific Research in Qiqihar University, China (Grant No. 2012k-Z02), and the Natural Science Foundation of Heilongjiang Province, China (Grant No. E201259).
The nanostructures during the tensile drawing of poly(ethylene terephthalate) (PET)/hexadecyl triphenyl phosphonium bromide montmorillonite (PMMT) nanocomposites were studied by in-situ small angle x-ray scattering. For strain higher than the yield point, the scattering intensity increases dramatically due to the nucleation and growth of nanovoids and crystals. The nanovoids and crystals are significantly dependent on the heating temperature. The effective filling of PMMT in the PET matrix provokes a strong restriction to the long period. The peaks of the long period disappear gradually with the deformation strain increasing from 0% to 34%.
Poly(ethylene terephthalate), PET, is a semi-crystalline thermoplastic polymer widely used in soft drink bottles, food and nonfood containers. The barrier property of PET to oxygen should be improved for the application of packaging such as soft drinks and beer. The PET nanocomposite reinforced with particular montmorillonite, MMT, becomes an important alternative to neat PET with improved mechanical and barrier properties because the reinforcement from the inorganic layers occurs in two dimensions. [ 1 – 4 ] The nanocomposite properties depend on the delamination of MMT nanoparticles into the PET matrix. Normally, the surface organic modification of MMT is recognized as an effective way to improve the composite compatibility. [ 5 , 6 ]
The understanding of the mechanisms behind the enhancement, as well as the effect of the MMT nanofiller on the structural evolution, is crucial for the successful industrial application of PET nanocomposites. In this regard, research efforts have been devoted to the nanostructures and the deformation mechanism characterizations of the PET polymer and PET/MMT nanocomposites during stretching with small angle x-ray scattering (SAXS) technique. [ 7 – 17 ] At small strains, SAXS showed no hierarchical structure in amorphous PET polymer. At intermediate strains, SAXS indicated the formation of a layered structure and a fibrillar domain on a large scale. At high strains, the crystal reorientation and lateral crystal growth took place. [ 11 ] Tensile deformation could induce a mesophase structure, which is the precursor to crystallization. Todorov et al. assessed the evolution of craze-like structures and void sizes in PET/MMT nanocomposites with in-situ SAXS experiments. The evolution of the PET/MMT nanocomposite multiscale structure has three main common stages. [ 7 ]
This work aims at a better understanding of the inter-relationships between hexadecyl triphenyl phosphonium bromide montmorillonite (PMMT) nanoparticles incorporated into a PET matrix and the nanostructure during stretching at room temperature and 90 °C above the glass transition temperature by using in-situ SAXS method. PMMT is prepared, and as a result, the compatibility of MMT is improved to a great extent. In addition, a new monomer is also synthesized by a condensation reaction between succinic anhydride and p-phenylene diamine to improve the barrier property and depress the crystallization of the PET/PMMT nanocomposites. The in-situ SAXS measurement yields the information about the nanostructure changes, including the scattering intensity, nanovoids, crystals, and long period. The observed nanostructure characteristics are highly consistent with the mechanical behaviors.
PMMT was obtained through a common preparation method. [ 18 , 19 ] Pure terephthalic acid (PTA) and ethylene glycol (EG) were purchased from Tokyo Kasei Kogyo Co. The succinic anhydride and p-phenylene diamine were supplied by Sinopharm Chemical Reagent Corp., and they were all of analytical purity.
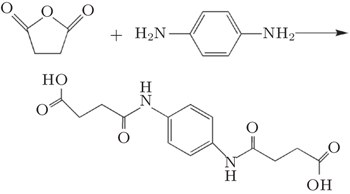
The new monomer was synthesized by a condensation reaction of succinic anhydride and p-phenylene diamine. A typical procedure for preparing the new monomer was as follows. 2.1 g p-phenylene diamine was dissolved in acetone. The mixed solution was added to another solution prepared by dissolving 4 g succinic anhydride in 40 mL acetone. The solution was then stirred at 600 rpm for 4 h at room temperature. The new monomer was purified by recrystallizing several times. The product was dried in a vacuum to extract the remaining solvent and non-reacted monomers. The preparation method of the new monomer was shown in Fig.
PTA and EG with weight ratios in the range of 1.14–3.0 were added into a 50-L reactor with 1 wt.% the new monomer. The reactor was quickly heated to 230–260 °C for the esterification reaction under nitrogen atmosphere at a pressure in the range of 0.3–0.5 MPa. Afterwards, PMMT was added into the reactor under a suitable viscosity and the polymerization was carried out at a temperature in the range of 275–280 °C and a pressure of 50 Pa. The compositions of the prepared PET/PMMT nanocomposites are presented in Table
| Table 1. Compositions of the prepared PET/PMMT nanocomposites. . |
The mechanical properties of the samples were examined using a Linkam TST350 tensile tester (Linkam Scientific Instruments, Surrey, UK) at room temperature and 90 °C above the glass transition temperature. The load cell and the crosshead speed used were 200 N and 10 μm/s, respectively. The specimens were cut into dog bone shape using a cutter. The test specimens possessed dimensions of 35 mm × 5.1 mm × 0.3 mm.
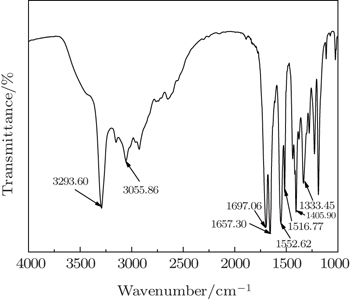
Fourier transform infrared (FTIR) spectroscopic analysis of the secondary-modified MMT was conducted on a Bruker Tensor 27 infrared spectrometer (Germany). For DSC (Q2000 TA) testing, samples of all resulting nanocomposites were subjected to heating from room temperature to 285 °C with the same rate of 10 °C/min. The scanning electron microscope (SEM) (Hitachi S-4300, Japan) was used to record and analyze the brittle fracture surface obtained in liquid nitrogen.
The small angle x-ray scattering experiment was performed at beam line 1W2A of Beijing synchrotron radiation facility (BSRF) with incident x-ray of wavelength 0.154 nm. The storage ring was operated at 2.5 GeV with a current about 200 mA. A Pilatus detector with 981 × 1043 pixels (pixel size: 172 μm × 172 μm) was positioned perpendicularly to the incident beam with a detector–sample distance of 1330 mm.
In-situ SAXS measurements were carried out during tensile tests. The pattern recording time of 10 s was chosen in order to have the best compromise between pattern resolution and minimum strain increment during detecting. The tensile stretching device can move both grips in opposite directions, so the x-ray beam can be maintained at the center of the PET/PMMT nanocomposites during the stretching procedures. The samples were mounted perpendicularly to the incident x-ray beam and stretched in the horizontal direction. Background scattering was subtracted and all scattering intensities were normalized with respect to the incident x-ray intensity.
Figure
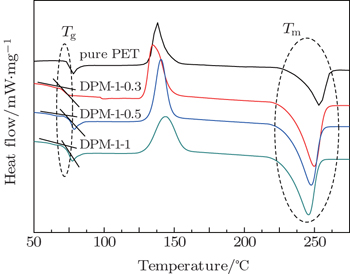
The DSC samples of all resulting nanocomposites are subjected to heating from room temperature (RT) to 285 °C with the same rate of 10 °C /min. Figure
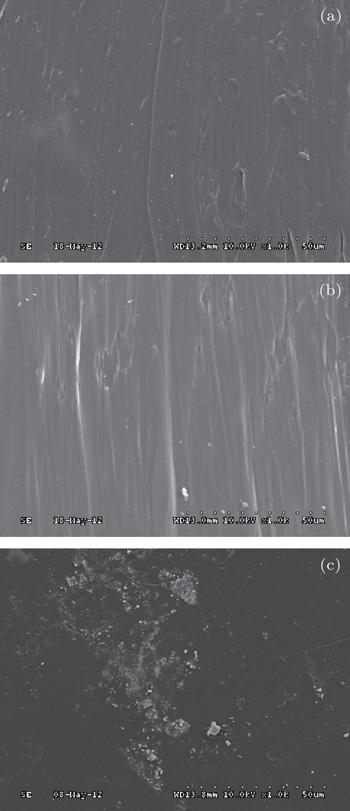
The SEM is used to characterize the PMMT nanoparticle distribution and to assess the presence of large aggregates. Figure
As is well known, the PMMT content affects the mechanical properties and microstructure of the PET/PMMT nanocomposites. Figure
Figure
 | Fig. 5. Stress–strain curves of the PET/PMMT nanocomposites as a function of the PMMT content at different temperatures. |
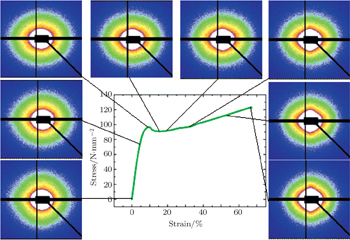 | Fig. 6. Selected SAXS patterns of DPM-1-1 along with the stress–strain curve during tensile stretching at room temperature. |
The SAXS patterns can be used to detect nanovoids. It may be useful to analyze the maximal intensities in the scattering patterns as a function of strain. The intensities should quickly increase from the moment when the nanovoids are formed. The integrated SAXS profiles of the PET/PMMT nanocomposites with strains are shown in Fig.
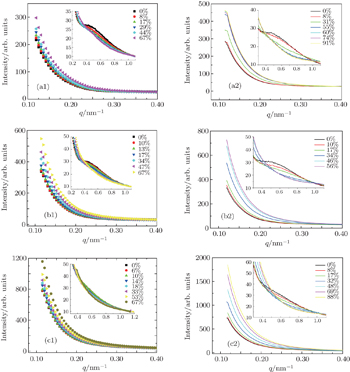 | Fig. 7. The SAXS profiles of nanocomposites (a1), (a2) DMP-1-0.3; (b1), (b2) DMP-1-0.3; and (c1), (c2) DMP-1-1 during stretching at RT and 90 °C, respectively. |
From the evolution of the shoulders, it can be speculated that the nanoparticles have a long period of layered nanostructure in the nanocomposites. On the SAXS intensity representing the scattering from the PET/PMMT nanocomposite, only one maximum of intensity, resulting from the periodic structure, is usually visible. This maximum is used to determine the long period of structure based on L = 2 π / q , where L is the long period and q is the scattering vector. [ 11 ] The correlation of the long period with the strain is shown in Fig.
In-situ SAXS experiments coupled with stretching were performed to investigate the nanostructure evolution of the PET/PMMT nanocomposites at RT and 90 °C. The SAXS appears as a very suitable technique to characterize the complex plastic deformation behavior. The load–strain curves at RT can be divided into different ranges of strain based on the SAXS experimental results and the nanostructure characteristics. Below about 10% strain, the scattering patterns are approximately isotropic and the scattering intensities have no obvious change. At above 10% strain (the yield point), an anisotropic SAXS pattern appears and the orientation becomes obvious with stretching along the meridional direction. It is conceivable that nanovoids and deformation-induced crystals are formed. The nanovoids elongate with increasing strain. Some PMMTs are oriented along the pulling axis and a layered nanostructure is formed inside the matrix of the PET/PMMT nanocomposites. At strain from 0% to about 34%, the long period of the layered nanostructure in the nanocomposites decreases with increasing PMMT content and deformation strain.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 |