† Corresponding author. E-mail:
Project supported by the National High Technology Research and Development Program of China (Grant No. 2015AA034201) and the Chinese Universities Scientific Fund (Grant No. 2015LX002).
Progress in electrochromic lithium ion batteries (LIBs) is reviewed, highlighting advances and possible research directions. Methods for using the LIB electrode materials’ magnetic properties are also described, using several examples. Li 4 Ti 5 O 12 (LTO) film is discussed as an electrochromic material and insertion compound. The opto-electrical properties of the LTO film have been characterized by electrical measurements and UV–Vis spectra. A prototype bi-functional electrochromic LIB, incorporating LTO as both electrochromic layer and anode, has also been characterized by charge– discharge measurements and UV–Vis transmittance. The results show that the bi-functional electrochromic LIB prototype works well. Magnetic measurement has proven to be a powerful tool to evaluate the quality of electrode materials. We introduce briefly the magnetism of solids in general, and then discuss the magnetic characteristics of layered oxides, spinel oxides, olivine phosphate LiFePO 4 , and Nasicon-type Li 3 Fe 2 (PO 4 ) 3 . We also discuss what kind of impurities can be detected, which will guide us to fabricate high quality films and high performance devices.
Lithium ion batteries (LIBs) have attracted a great deal of attention in recent decades due to their promise for hybrid vehicle applications, power supplies for portable equipments, etc. [ 1 ] Much effort has been expended to improve LIB performance dimensions such as long term stability and high power/charge capability, which are both highly dependent on the quality of the electrode and electrolyte materials. The electrode material of an LIB is usually an insertion compound, which provides pathways for shuttling lithium ions, benefitting both fast ion transport and charge balance. Insertion electrode materials used for LIBs include layered oxides with α -NaFeO 2 -type structure, spinel oxides, and olivine phosphates. [ 2 ] Keep in mind, however, that insertion electrode materials (which include electrochromic metal oxides), LIBs, and conducting polymers [ 3 , 4 ] are developed not only for energy storage applications, but also for electrochromic use.
Very recently, an exciting research report entitled “bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery applications” was published in Nature Communications ; [ 5 ] it provides the possibility of combining electrochromism and a secondary battery to realize the dual functions of electrochromism and energy storage on the same device. Although that work is in an early stage, scientists are inspired to combine electrochromism and LIBs through finding insertion electrode materials that change color at an applied voltage/electric field. This kind of work is likely to become a new research hotspot in the scientific community in the near future.
The combination of electrochromism and LIBs requires matching electrode materials. For example, both electrodes must be insertion materials that can intercalate/de-intercalate and store lithium, and at least one electrode exhibits electrochromism; redox on both electrodes should be performed at the same time in order to maintain charge balance in the device and circuit; matching the electronic conductivity and lithium ionic conductivity of the electrode materials is another important issue.
The bi-functional devices combining electrochromism and LIBs require high quality electrode materials to maximize their opto-electrical properties and lifetime. Magnetic experiments are powerful tools to study the fundamental properties of lithium intercalation compounds and to assess their quality, [ 2 ] because impurities in electrode materials exert significant influence on the magnetic properties. In addition, observing the effects of temperature and stress on the magnetic properties reveals where the properties originate and how the electrons are configured. Cherneva et al . demonstrated how the use of magnetic properties can advance the structure, composition, and electrochemical performance of LIB materials. [ 6 ] For instance, the magnetic properties are highly sensitive to structural defects and impurities, often beyond the sensitivity threshold of x-ray diffraction. In this review, we introduce the mainstream of progress in researching bi-functional devices that combine electrochromism and LIB energy storage, highlighting the advances and the possible research directions. We also describe, using several examples, how to use the LIB electrode materials’ magnetic properties.
Electrochromism (EC) is the reversible change of optical absorption bands in materials, perceived as a reversible change in color, when a positive or negative bias is applied, accompanied by ion-transfer and redox processes on positive and/or negative electrodes. Thanks to Deb’s early work, [ 7 ] electrochromism has already attracted a great deal of attention in the scientific community. Two of the most suitable inorganic EC materials, WO 3 and NiO, have been studied extensively. [ 8 , 9 ] Among organic EC materials, poly (3, 4-ethylenedioxythiophenes) (PEDOTs) are currently considered to be most suitable for applications on microfiber textile substrates. [ 10 ] EC materials are used for fabricating large-area smart windows, which were first proposed by Lampert and Granqvist in the mid 1980s. [ 11 , 12 ]
A conventional electrochromic device (ECD) consists of a sandwich configuration of electrodes, necessitating the use of at least one optically transparent electrode, such as indium tin oxide. This type of ECD, in its simplest configuration, is made of three layers of electrochromic materials deposited in the form of thin films on a glass or polymer substrate. The middle layer is an ionic conductor (electrolyte): it loses ions when an electric current flows through it at relatively low voltage (1–5 V). The electrolyte layer is positioned between two other layers: an electrochromic film, which acts as the electrode, and a layer for the accumulation of electrons (counter electrode).
However, an electrochromic device usually consists of five layers in order to obtain charge balance and long term stability. The first one is a transparent electron conductor deposited on glass or plastic for the purpose of collecting or providing electrons uniformly throughout a large area. The second one is a layer of electrochromic materials, capable of transferring electrons and ions simultaneously. The third one is an ion conductor or electrolyte that conducts ions but not electrons. The fourth layer is an ion storage layer, made of insertion materials or even other EC materials in order to accommodate ions from the third/second layers. The fifth layer is a transparent electron conductor or metallic films for collecting or providing electrons uniformly in a large area.
A particular class of electrochromic materials is metal oxides and lithiated metal oxides; they have been used as electrodes of lithium ion batteries. Electrochromic devices based on the electrodes of lithium ion batteries and the five layer structure described above must be charge balanced. That is, every electron that enters the device from an external circuit through an electrode must be compensated by one electron leaving the other electrode. At the same time, the electrolyte between them transfers ions fast but blocks electrons strongly.
Li 4 Ti 5 O 12 (LTO) is a “zero-strain” insertion material that can be used as the electrochromic layer and/or the anode of a lithium ion battery or a sodium ion battery. [ 13 – 15 ] Recently, electrochromism in LTO film was reported by Yu et al. [ 16 ] In that work, an LTO thin film electrode was deposited at 600 °C on fluorine-doped tin oxide glass. A three-electrode cell was constructed using the LTO thin-film electrode as the working electrode and lithium foils as the counter and reference electrodes, respectively. The electrolyte was 1 M LiPF 6 dissolved in ethylene carbonate (EC) and dimethyl carbonate with a volume ratio of 1:1 (Shanghai Topsol Ltd., H 2 O < 10 ppm). It was found that the response times of LTO coloration and bleaching, as shown in Fig.
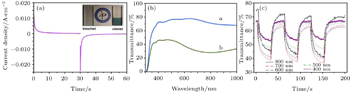 | Fig. 1. (a) Current density–time curves acquired by potential step measurement applied between 1.2 V and 3 V. The inset shows optical images of the electrochromic effect in the Li 4 Ti 5 O 12 /FTO electrode at bleached and colored states. (b) Transmittance spectra of the Li 4 Ti 5 O 12 /FTO electrode at (i) bleached and (ii) colored states. (c) In situ transmittance curves obtained during switched pulse potential cycling tests with wavelengths of 850 nm, 700 nm, 600 nm, 500 nm, and 400 nm. [ 16 ] |
From Fig.
Very recently, Nagai et al. developed a bi-functional device with an LTO thin film anode (90 nm in thickness) and a transparent thin film Li 3 Fe 2 (PO 4 ) 3 (LFP) cathode. [ 18 ] Both thin-film electrodes were fabricated on fluorine-doped tin oxide (FTO). The assembled sandwich-type LIB incorporated a solution containing LiPF 6 as its electrolyte. The assembled LIB was repeatedly charged/discharged successfully at a constant current of 10 μA, and the curve of the voltage is shown in Fig.
 | Fig. 2. (a) Repeated charge/discharge test of the battery. The charge at the constant current of 10 μA and spontaneous discharge was repeated at 20 s intervals. (b) The colorless battery before charge and after discharge (top) and blue-gray battery after charge (bottom). (c) UV–Vis spectra of the battery before charge (solid line), after charge, and after discharge (dash line). [ 18 ] |
When the battery was charged from an external source, the colorless battery drastically changed color to blue-gray, as shown in Fig.
The electrochromic LIB developed by Nagai et al . is a significant bi-functional prototype device. Nevertheless, the use of a liquid electrolyte would hinder applications of the electrochromic LIB as a large-area smart window and/or thin film microdevice because of possible leaks and because of electrolyte instability under UV illumination. To overcome the problem, it is necessary to use a solid state electrolyte instead in electrochromic LIBs, even though the ionic conductivity of liquid electrolytes is about two orders of magnitude greater than that of the conventional solid state electrolytes. With the development of research on solid state electrolytes, this difference is being changed. The solid state electrolytes developed recently, such as Li 2 S–P 2 S 5 , [ 20 – 22 ] Li 1.07 Al 0.69 Ti 1.46 (PO 4 ) 3 , and Li 1.5 Al 0.5 Ge 1.5 (PO 4 ) 3 , have high ionic conductivity close to that of liquid electrolytes, so they are suitable electrolytes for solid state LIBs. More importantly, some electrolytes have a wide electrochemical window, exceeding 5 V, which is very suitable to combine with high voltage cathodes such as LiNi 0.5 Mn 1.5 O 4 [ 23 ] and anodes made of LTO, leading to an output of 3 V from an electrochromic LIB system. Therefore, all-solid state electrochromic LIBs will be a research direction in the immediate future.
Magnetism in solid materials stems mainly from electron motion, because the magnetic moment of electrons is much greater than that of their nuclei. When a solid material is put in a magnetic field with the intensity H , the magnetization M is defined as the magnetic moment per unit volume, and the magnetic susceptibility per unit volume is defined as χ = ∂M / ∂H ; it can be simplified to χ = M / H in the case of a linear M ( H ). Quite frequently, the susceptibility is defined referred to unit mass or to a mole of substance. [ 24 ] Molar susceptibility is written as χ m . Materials may be classified into five categories with regard to χ m , i.e., diamagnetic, paramagnetic, ferromagnetic, ferrimagnetic, and antiferromagnetic. If χ m is positive, the material is called paramagnetic, and a magnetic field is strengthened by the presence of the material. If χ m is negative, the material is diamagnetic; accordingly, a magnetic field is weakened by the presence of such materials. If a spontaneous magnetic moment exists even in zero applied magnetic field, this suggests that electron spins and magnetic moments are arranged in a regular manner. In an antiferromagnet, the spins are ordered in an antiparallel arrangement with zero net moment at temperatures below the ordering or Neel temperature. The susceptibility of an antiferromagnet is not infinite at T = T N but exhibits a weak cusp. Figure
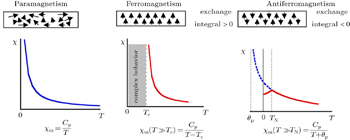 | Fig. 3. Temperature dependence of the magnetic susceptibility of paramagnetic, ferromagnetic, and antiferromagnetic solids. [ 2 ] |
Paramagnetic materials attract and repel like normal magnets in response to an applied magnetic field. There are three types of paramagnetism: Van Vleck paramagnetism, Pauli paramagnetism, and Langevin paramagnetism. The first two are temperature independent. Pauli paramagnetism is suitable to describe the magnetism of metals. Van Vleck paramagnetism occurs when the energy difference between the excited state and the ground state with no magnetic moment significantly exceeds the thermal energy. Langevin paramagnetism, however, is temperature dependent, described by the Curie law χ = C / T and the Curie–Weiss law χ = C /( T + θ p ), and found in compounds containing unpaired electrons, such as those of iron group ions and trivalent lanthanide group ions. [ 24 ] Here C is the Curie constant, and the Weiss constant θ p typically accounts for magnetic ordering of the electronic moments below the Curie or Neel temperature for uncorrelated spins. For a paramagnet having an effective moment μ eff , the molar Curie constant is written as 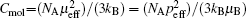
When the orbital angular momentum is quenched ( L = 0, J = S ), which commonly occurs for transition metal ions from the iron group, the theoretical p eff = 2[ S ( S + 1)] 1/2 , where S refers to the electronic spin quantum number.
When the Curie constant is determined experimentally by fitting the linear 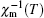
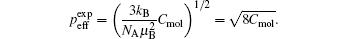
Comparing the theoretical and experimental p eff values, one can deduce the electronic configuration of the magnetic ions of the iron group.
For magnetic ordering materials, including iron, nickel, cobalt, and manganese, the Curie–Weiss law χ = C /( T + θ p ) is used to distinguish ferromagnetic versus antiferromagnetic through θ p , which contains information on the strength and type of interactions between the magnetic moments, known as magnetic exchange. If the magnetic moments are well separated spatially and cannot interact, then θ p = 0 K, and the Curie–Weiss law is simplified to the Curie law. Negative θ p indicates a ferromagnetic exchange, which tends to align the magnetic moments in parallel. Positive θ p indicates an antiferromagnetic exchange. However, Julien et al. reported that θ p in antiferromagnetic materials can be either positive or negative, depending on the type of antiferromagnetic interaction.
Although the absolute value of θ p is called the paramagnetic Curie temperature, it should be emphasize that θ p is not the Neel temperature ( T N ) above which antiferromagnetism becomes paramagnetism. One can obtain T N from the cusp of the χ ( T ) curve. On the other hand, θ p is obtained from the χ −1 ( T ) curve by linearly extrapolating the paramagnetic component to 1/ χ = 0, yielding an intercept − C / χ 0 on the T axis, where χ 0 is T -independent and consists of a diamagnetic ( χ dia ) contribution and an orbital ( χ orb ) contribution. [ 25 ] Fortunately, one can alternatively obtain T N from the cusp of the χ −1 ( T ) curve.
The conventional measurement of magnetism includes the temperature and magnetic field dependencies of the magnetization. Temperature dependence reveals the transition points of magnetism, such as the Curie point and the Neel temperature, and it determines where magnetic ordering occurs. Temperature dependence may be observed through a superconducting quantum interference device (SQUID) magnetometer in magnetic fields. The magnetic field dependence can show the net magnetic moment formation upon ordering. Considering temperature and magnetic field dependences together, one can obtain the temperature dependence of field cooled (FC) and zero-field cooled (ZFC) curves under 100 Oe by employing a vibration sample magnetometer (VSM) to find more detail on the nature of the magnetic transitions.
The magnetism of electrode materials for LIBs is related to metallic ions and to the crystal lattice in which the ions are arranged. The structures of electrode materials are classified into three main types: layered oxides, e.g., LM O 2 ( M = Co, Ni, Mn) with the α -NaFeO 2 structure; spinel oxides, e.g., Li 4 Ti 5 O 12 , LiNi 0.5 Mn 1.5 O 4 ; and olivine-phosphates, e.g., LiFePO 4 . In addition, the magnetism of Nasicon-type Li 3 Fe 2 (PO4) 3 was studied in many research groups.
Magnetic properties of many layered oxides with the α -NaFeO 2 -type structure, such as LiCoO 2 and LiNiO 2 , have been studied. The structure–magnetism relationships of sodium and lithium ferrites have been investigated to evaluate detectable ferromagnetic impurities and defects. [ 26 , 27 ] In the case of LiNiO 2 , the difficulty of obtaining stoichiometric LiNiO 2 is well known. In order to estimate the excess Ni in the Li plane of LiNiO 2 Sugiyama et al. investigated the susceptibility measured below 400 K under an H ≤ 10 kOe field with a SQUID (MPMS, Quantum Design), [ 28 ] and determined the Weiss temperature and the effective magnetic moment to be θ p = − θ cw = −40.8± 0.5 K and μ eff = 2.14 ± 0.01 μ B , respectively, by fitting to the Curie–Weiss law. Since the magnetic properties are extremely sensitive to the Ni 2+ contribution, their use is recommended to evaluate the stoichiometric deviation in Li 1− x Ni 1+ x O 2 . The characterization of magnetic properties for Li 1− x Ni 1+ x O 2 is a powerful tool to evaluate the quality of electrode materials for electrochromic LIBs, although the types of magnetism at play in this electrode material are controversial. [ 29 – 36 ]
The magnetic properties of AB 2 O 4 spinel oxides depend strongly on the cation distribution between A and B sites. When both sites contain spin-bearing cations, ferrimagnetism is often observed arising from antiferromagnetic interactions between the A and B sublattices. [ 37 ] When the A site contains diamagnetic cations alone, the spinel structure gives rise to magnetic frustration, since the B sublattice has the “pyrochlore network” topology with cations occupying the apices of corner-sharing tetrahedra. [ 38 ] In Li 4 Mn 5− x Ti x O 12 systems the A site contains diamagnetic lithium alone (4–5–12 system) or a small fraction of transition metal. The composition of the B site, however, is rather complex, since it contains spin-bearing cations such as Mn 4+ and Ni 2+ , partially diluted by diamagnetic cations Li + and Ti 4+ . This dilution is even more prominent in the 4–5–12 system, because of the presence of lithium on the B site.
The temperature dependence of the inverse molar susceptibility χ m of Li 4 Mn 5− x Ti x O 12 samples is shown in Fig.
| Table 1. Magnetic parameters of Ti/Mn substituted spinel. [ 39 ] . |
 | Fig. 4. Temperature dependence of the inverse magnetic susceptibility of members of the Li 4 Mn 5− x Ti x O 12 series in a magnetic field of 100 Oe (all data per 4–5–12 formula unit). [ 39 ] |
Amdouni et al. reported the magnetic properties in LiNi 0.5 Mn 1.5 O 4 (LNMO) spinel prepared by wet chemical methods. [ 40 ] Figure
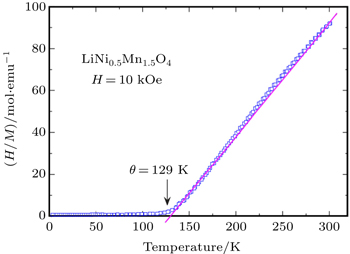 | Fig. 5. Temperature dependence of reciprocal molar magnetization normalized by magnetic fields ( H / M ) under an applied field H = 10 kOe for LiNi 0.5 Mn 1.5 O 4 spinel grown by the wet-chemical route. Ferromagnetic ordering is clearly shown at the Curie temperature θ = 129 K for the Ni-doped spinel. [ 40 ] |
High quality LNMO with only infrequent impurities is a crucial component of bi-functional electrochromic LIBs. Measurement of saturation magnetization at very low temperature is a better way to detect the amount of impurities in LNMO, even beyond the detection limit of XRD. [ 40 ]
Olivine-type LiFePO 4 and Nasicon-type Li 3 Fe 2 (PO 4 ) 3 have been proposed as novel electrode materials for LIBs. [ 45 – 47 ] In order to know the macroscopic magnetic properties of LiFePO 4 , its susceptibility was measured below 400 K under an H ≤ 10 kOe field with a SQUID (MPMS, Quantum Design), [ 48 ] as shown in Fig.
 | Fig. 6. Temperature dependence of inverse susceptibility (1/ χ ) for LiFePO4. The χ data were obtained in field cooling (FC) mode with H = 10 kOe. A solid line represents a linear fit in the temperature range between 100 K and 400 K using the Curie–Weiss formula. [ 48 ] |
Nasicon-type Li 3 Fe 2 (PO 4 ) 3 , compared with LiFePO 4 , has a relatively high electric conductivity, resulting from the disorder of lithium ions in the structure. It has been suggested that Nasicon-type crystals such as Li 3 Fe 2 (PO 4 ) 3 can be used as electrolytes. It should be noted that the valences of iron ions in Li 3 Fe 2 (PO 4 ) 3 and LiFePO 4 are Fe 3+ and Fe 2+ , respectively. [ 53 ] Therefore, the magnetic properties of these two materials are expected to differ. Figure
 | Fig. 7. Temperature dependence of magnetization under 100 Oe between 4.2 K and 295 K. [ 54 ] |
In this paper, progress on two of the most exciting and promising research topics in the field of LIB electrode materials electrochromism and magnetism has been reviewed. As we move forward in the design of devices, electrochromic materials and LIB electrode materials must both be insertion materials. Electrochromic LIBs must consist of five layers for the purpose of charge balance. The possibility of bi-functional devices that use LTO as the EC layer has already been demonstrated. A prototype bi-functional electrochromic LIB based on a liquid electrolyte has been demonstrated. Thanks to the progress of solid state electrolyte research, high ionic conductors can be obtained; therefore it may be expected that successful all-solid state bi-functional electrochromic LIBs will be fabricated in the near future. The opto-electrical properties and long term stability of bi-functional electrochromic devices depend heavily on the quality of both electrode film materials and solid state electrolytes. Measuring changes in the magnetic parameters of LIB electrode materials is effective in detecting impurities and structural changes in layered oxides, spinel oxides, olivine phosphates, or Nasicon-type Li 3 Fe 2 (PO 4 ) 3 . For these reasons, we feel confident that with the ongoing improvement of magnetic properties in electrode materials, the quality of films also improves, carrying high performance bi-functional electrochromic devices toward widespread application.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 |


