† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the Supercomputing Center of Chinese Academy of Sciences in Beijing, China, the Shanghai Supercomputer Center, China, the National Natural Science Foundation of China (Grant Nos. 21273268, 11290164, and 11175230), the Startup Funding from Shanghai Institute of Applied Physics, Chinese Academy of Sciences (Grant No. Y290011011), “Hundred People Project” from Chinese Academy of Sciences, and “Pu-jiang Rencai Project” from Science and Technology Commission of Shanghai Municipality, China (Grant No. 13PJ1410400).
Anesthetics are extremely important in modern surgery to greatly reduce the patient’s pain. The understanding of anesthesia at molecular level is the preliminary step for the application of anesthetics in clinic safely and effectively. Inert gases, with low chemical activity, have been found to cause anesthesia for centuries, but the mechanism is unclear yet. In this review, we first summarize the progress of theories about general anesthesia, especially for inert gas narcosis, and then propose a new hypothesis that the aggregated rather than the dispersed inert gas molecules are the key to trigger the narcosis to explain the steep dose-response relationship of anesthesia.
It has long been accepted that the nanoparticles hardly enter the blood circulation system and tissues of living animals, let alone the cells. Thus, most of the previous experiments are in vitro or focus on the damage to the lung and gastrointestinal tract, or even directly inject the nanoparticles into the blood vessel of living animals. [ 1 – 13 ] In contrast, the small gas molecules can easily dissolve into the blood in alveolus with our breathing. When the partial pressure of gases increases, the concentration of gas molecules dissolved in blood increases.
According to the effects to animals, gases can be divided into three kinds roughly. The first kind of gases can participate in the metabolism (oxygen) to generate the metabolic products (carbon dioxide, ethanol) or regulate the physiological processes (Nitric oxide). [ 14 ] In normal physiological level, these gases are harmless to body. The second kind of gases can react with the components of living body and cause damage to cells or even lead to the death of living creatures (i.e., carbon monoxide, ammonia gas, hydrogen sulfide, benzene, formaldehyde, and so on). The third kind of gases is the biological inert gas. Biological inert gases mean they are all inert biologically, and exert their effects without changing their own chemical structures or the primary chemical structures of biological tissues, including helium, neon, argon, krypton, xenon, hydrogen, nitrous oxide, and so on. Nearly all the biological inert gases at high partial pressure can cause anesthesia. [ 15 ] In this paper, we will briefly review the history of anesthesia, especially inert gas narcosis, and the developing of the theories about general anesthesia. We also present our hypothesis that the inert gases may exert their effects as clusters, which can explain some difficulties explained hardly by previous theories.
Nitrogen narcosis has been well known for centuries. In 1835, Junod noted that when breathing compressed air ‘the functions of the brain are activated, imagination is lively, thoughts have a peculiar charm and, in some persons, symptoms of intoxication are present.’ [ 16 ] While diving at depth more than 30–40 m, the divers inhale compressed air and will be involved into nitrogen narcosis, like drinking alcohol. [ 15 ] The degree of nitrogen narcosis will increase along the depth increasing and will make the diving at depth more than 40 meter a dangerous behavior.
In 1844, Horace Wells used nitrous oxide in dental surgery to relax patients. [ 17 ] This is the first time for the inert gas to be used as anesthetic in history. Until nowadays, the nitrous oxide is still the unique gas anesthetic often used in surgeries. Due to the high concentration (105%) needed by nitrous oxide anesthesia, it usually mixed with other anesthetics when used in surgeries.
Xenon was discovered by Ramsay and Travers in 1898. It is very rare in nature and only constitutes 0.0000087% of the atmosphere. [ 18 ] When xenon is account for 71% of inhaled gases, human will lost consciousness. [ 19 ] The blood/gas partition coefficient of xenon is only 0.115, which means xenon is the least soluble gas that can be directly used for anesthesia. [ 20 ] Xenon was shown to have anesthetic properties in mice in 1946, [ 21 ] and Stuart Cullen and Erwin Gross anesthetized two patients with it in 1951. [ 22 ] Although the price of xenon limits its widespread application in anesthesia, scientists still think it is the anesthetic for the 21st century. Compared to other anesthetics commonly used in anesthesia, xenon is totally inert to our body, which means that it nearly does not cause side effects. [ 23 ] Because of its low solubility in blood, the unconsciousness and weakness for xenon anesthesia is very rapid.
The strengths of anesthesia for different kinds of inert gases vary greatly. To determine the narcotic potency of anesthetics quantitatively, Eger et al. proposed a technique, called MAC (minimum alveolar concentration). [ 24 ] MAC is the partial pressure (concentration) of the vapor in the lungs that is required to prevent movement (motor response) in 50% of subjects in response to surgical (pain) stimulus. MAC was found to be fairly constant for any subject. The narcotic potency is defined as the reciprocal of MAC (1/MAC). The relative narcotic potency is the relative value when we set the narcotic potency of nitrogen as 1.
Along with the application of anesthetics in clinic, the exploration of the mechanism of anesthesia never stops. In history, two main theories were proposed to explain the mechanism of inert gas anesthesia, the lipid hypotheses and the protein hypotheses.
Early in 1847, von Bibra and Harless proposed the first hypothesis of anesthesia. [ 25 ] They thought the anesthetics dissolved into the fat fraction of brain cells and caused the anesthesia. From 1899 to 1901, Mayer and Overton reported their experimental findings in succession. [ 26 – 29 ] They compared the concentrations required to induce anesthesia of several kinds of anesthetics and their olive oil/water partition coefficient and found that for kinds of anesthetics it is a nearly inversely proportional relationship between them. Figure
 | Fig. 1. The linear correlation between the narcotic potency and the oil/water partition coefficient for some inert gases. Data from Ref. [ 15 ]. |
In the following 80 years, many scientists proposed their theories according to the Mayer–Overton correlation. They thought the anesthetics might (i) change bilayer dimensions (volume or thickness), [ 30 , 31 ] (ii) increase bilayer fluidity, [ 32 ] (iii) induce phase transitions in bilayer lipids, [ 33 , 34 ] (iv) alter bilayer permeability. [ 35 ] However, up to now, the lipid hypotheses have been discarded. The lipid hypotheses encountered troubles to explain the findings in experiments. There are some objections to Lipid hypotheses. The direct objection is that detectable changes in bilayer structure cannot be observed at clinically relevant concentrations in experiments. [ 36 ] Lipid hypotheses also predict that the MAC decreases with the rise of temperature. However, in fact the opposite is true. [ 37 , 38 ]
In 1984, Franks and Lieb found that the activity of a pure solute protein (firefly luciferase) can be inhibited by 50% at surgical level of halothane. [ 39 ] This finding inspired them that anesthesia might be caused by the direct binding of anesthetic molecules to proteins. After that, scientists turn their attention to the protein hypothesis of anesthesia. The most important question became that which proteins are the target proteins for anesthetics. Scientist have found that most general anesthetics can enhance the activity of inhibitory GAGA A (g-aminobutyric acid type-A) and glycine receptor. [ 40 , 41 ] But for different anesthetics, the target proteins might be also different. In 1998, Franks and his coworkers found in experiment that 80% xenon reduced NMDA-activated (N-methyl-Daspartate) currents by about 60%, but the effects of Xenon to GABA A receptors are negligible. [ 42 ]
However, the protein hypothesis is not perfect. An unexplained phenomenon for protein hypotheses is the steep dose-response relationship (Fig.
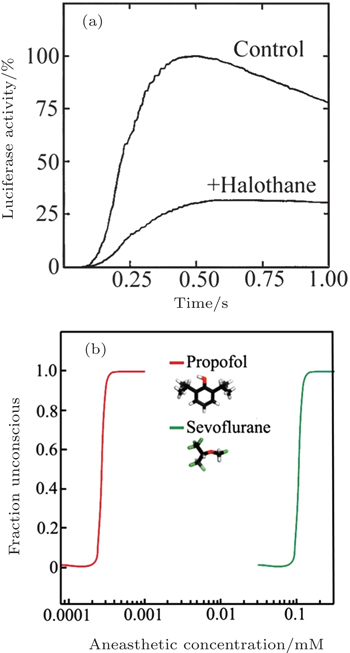 | Fig. 2. (a) Inhibition of luciferase activity at surgical levels of halothane. The curves show luciferase activity as a function of time. (b) The concentration–response curves for anaesthetic-induced loss of consciousness are extremely steep. Panel (a) is reprinted by permission from Nature Publishing Group Ltd: [Nature] (Ref. [ 39 ]) copyright (1984). Panel (b) is reprinted by permission from Nature Publishing Group Ltd: [Nature Reviews Neuroscience] (Ref. [ 42 ]) copyright (2008). |
Indeed, the aggregation of gas molecules in nanoscale has been observed experiments in recently years. In 2000, Lou et al. first reported the observation of nanobubble at the interface solid and water by atomic force microscopy. [ 44 ] Later, many more evidences from experiments have demonstrated the existing of nanobubble and some theories were proposed to understand the stability of nanobubbles. [ 45 , 46 , 57 ] Since 2006, Fang and his coworkers have proposed that the nanobubble may have a high density inside and the high inert density can enhance the life time of nanobubble. [ 58 – 60 ]
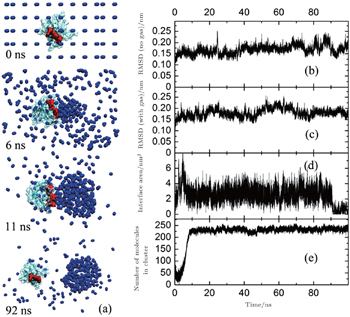
In 2013, we compared the affinities of nitrogen cluster and dispersed nitrogen molecules to SH3 domain (Fig.
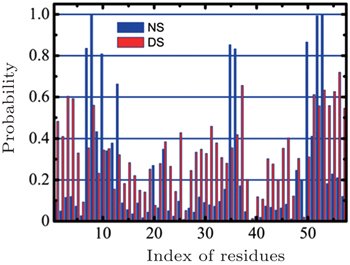 | Fig. 3. A comparison of the nitrogen cluster system and the dispersed nitrogen molecules system. The X -axis represents the index of residues in the SH3 domain while the Y -axis represents the probability of adsorption that residue adsorbed nitrogen molecules. Reprinted by permission from Nature Publishing Group Ltd: [Scientific Reports] (Ref. [ 43 ]) copyright (2013). |
In reality, when animals inhaled anesthetic with a concentration higher than MAC, they would lost consciousness and sensibility to pain. When the inhaled concentration of anesthetic decreased to lower that MAC, the animals can awake quickly. This fact suggests the process of anesthesia should be reversible, which means that the anesthetics cannot produce irreversible change to animal’s body. To show the influences of nitrogen cluster binding to the SH3 domain more clearly, we found a sample in which the cluster of nitrogen departed from the SH3 domain at last. In this sample, the nitrogen molecules aggregated quickly in 10 ns at the key binding sites (red part) of the SH3 domain binding to its ligand (Fig.
We calculated the RMSD (root mean square deviation, Fig.
In our recent work, we calculated the nucleation concentration of inert gas molecules in water by molecular dynamic simulation. Our results indicated that the narcotic potency might be determined by the ratio of the solubility of inert gas in water under 1 atm ( S ), the nucleation concentration of inert gas ( C ), and the adsorption probability of protein to inert gas molecules ( P ) (Fig.
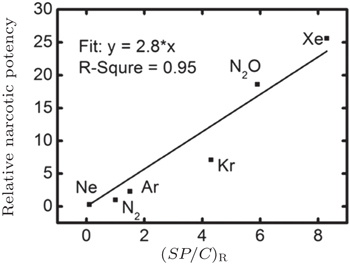 | Fig. 5. The linear correlation between relative narcotic potency [ 15 ] and ( SP / C ) R . |
The anesthetics have been used in clinic for more than a century. Until recent decades, the mechanisms of anesthesia have made great progress, although there are still problems hardly explained. We reviewed the history of anesthesia and the theories about general anesthesia, especially for inert gas molecules narcosis. We also proposed our hypothesis that the aggregated rather than the dispersed inert gas molecules are the causing factor to trigger the narcosis. By molecular dynamic simulation, we showed that the aggregated nitrogen molecules can adsorb on the hydrophobic area of the protein surface. In addition, if the inert gas molecules exert their effects as cluster state rather than dispersed molecules, some difficulties explained hardly by previous theories become understandable. The urgent need of our hypothesis is waiting for the direct experimental evidence. If it could be confirmed, it will enhance the safe application of inert gases in anesthesia and help to understand the molecular mechanism of anesthesia.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 | |
| 56 | |
| 57 | |
| 58 | |
| 59 | |
| 60 | |
| 61 | |
| 62 | |
| 63 | |
| 64 | |
| 65 |