†Corresponding author. E-mail: whwangnk@nankai.edu.cn
‡Corresponding author. E-mail: chengyahui@nankai.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 11104148, 51101088, and 51171082), the Tianjin Natural Science Foundation, China (Grant Nos. 14JCZDJC37700 and 13JCQNJC02800), the Specialized Research Fund for the Doctoral Program of Higher Education, China (Grant No. 20110031110034), and the Fundamental Research Funds for the Central Universities, China.
Cu xCu2O1− x (0.09 ⩽ x ⩽ 1.00) granular films with thickness about 280 nm have been fabricated by direct current reactive magnetron sputtering. The atomic ratio x can be controlled by the oxygen flow rate during Cu xCu2O1− x deposition. Room-temperature ferromagnetism (FM) is found in all of the samples. The saturated magnetization increases at first and then decreases with the decrease of x. The photoluminescence spectra show that the magnetization is closely correlated with the Cu vacancies in the Cu xCu2O1− x granular films. Fundamentally, the FM could be understood by the Stoner model based on the charge transfer mechanism. These results may provide solid evidence and physical insights on the origin of FM in the Cu2O-based oxides diluted magnetic semiconductors, especially for systems without intentional magnetic atom doping.
Oxide diluted magnetic semiconductors (ODMS) have attracted great attention due to their potential applications in spintronic devices.[1– 3] In the last two decades, room-temperature ferromagnetism (FM) has been observed in many ODMS systems, such as Mn: ZnO, [4] Co: TiO2, [5] Co: SnO2, [6] Cu: ZnO, [7] and etc. Interestingly, some metal oxide semiconductors have exhibited large intrinsic FM, even without intentional doping. Venkatesan et al. have reported room-temperature FM in undoped HfO2 and ZrO2 films.[8] Similar observations have been shown in low-dimensional structures of TiO2, In2O3, [9] ZnO.[10– 14] These findings have stimulated a debate on the physical mechanism of the FM in ODMS. Some believe that O defects are the origin of the FM, [10, 15] while others think that the FM is contributed by the cation defects in some systems.[16] Among these discussion on the FM in ODMS, it is worthwhile noting that the p-type related ODMS have drawn less attention than the n-type ODMS, like ZnO. However, the p-type materials are necessary to make p– n junctions. Therefore, the fabrication of p-type ODMS and further unveiling of their microscopic FM origin are significant in the current stage.[17, 18]
As a natural p-type oxide semiconductor, cuprous oxide (Cu2O) has been widely investigated in photovoltaic devices, [19] field effect transistors, [20] and photocatalysis.[21] Cu2O doped with transition metal (TM) atoms has demonstrated room-temperature FM.[22, 23] Interestingly, room-temperature FM has also been obtained in undoped Cu2O nanoparticles, fine powder, and nanowires.[24– 26] Both O defects and Cu defects are the possible origin of the FM in undoped Cu2O. However, it is rarely understood that which defect is dominant and how to modulate the FM through this defect.
In order to provide more experimental evidence on the FM in undoped Cu2O and to tune the FM with relatively simple fabrication technique, the CuxCu2O1− x (0.09 ⩽ x ⩽ 1.00) granular films without intentional magnetic dopants were prepared by reactive direct current (DC) magnetron sputtering. Room-temperature FM was observed in all samples. It is found that the saturated magnetization has a direct correlation with the oxygen flow rate. Actually, the oxygen flow rate determines the atomic ratio of Cu (x) in the granular films. Thus, the magnetization varies with x. Fundamentally, the origin of FM could be understood by the Stoner model based on the charge transfer mechanism.
CuxCu2O1− x granular films were deposited on the glass substrates, and Cu metal (99.99%) was used as the target. The base pressure of the vacuum chamber was below 5× 10− 5 Pa before CuxCu2O1− x deposition. During the sputtering, the pressure of chamber was kept at 0.8 Pa under Ar and O2 mixed ambient, the sample holder was rotated at 20 cycles per minute and the distance from the substrate to the Cu target was 12 cm.
The samples were prepared at room temperature and the DC magnetron sputtering power was kept at 90 W, while the oxygen flow rate was controlled within 0– 5.0 sccm. In this way, samples with different ratios x were prepared. The thickness (about 280 nm) of the samples was measured by profilometer (Dektak 6M). The structural information was characterized by transmission electron microscopy (TEM) (Tecnai G2 F20). The composition of the films was analyzed by x-ray photoelectron spectroscopy (XPS) (Perkin-Elmer PHi 5600). The magnetic properties of the samples were measured by Physical Property Measurement System (PPMS-9). The optical properties measurements were performed with spectrophotometer (UV-Visible/NIR, U-4100, Hitachi Inc.) for absorption spectra and spectrofluorometer (FL3-2-IHR221-NIR-TCSPC, HORIBA Jobin Yvon Inc.) for photoluminescence (PL) spectra.
Figure 1 shows the TEM images and the corresponding electron diffraction patterns of the samples prepared under the oxygen flow rates of 2.0 sccm, 3.0 sccm, 3.4 sccm, and 4.0 sccm, respectively. In Fig. 1(a), both Cu and Cu2O phases can be clearly observed according to the electron diffraction pattern, which indicates that the granular film is composed of Cu and Cu2O. The diffraction pattern of Cu fades away, while that of Cu2O brightens gradually with increasing the oxygen flow rate as shown in Figs. 1(b) and 1(c). This tendency implies that the ratio of Cu2O is enhanced in the granular films. When the oxygen flow rate reaches 4.0 sccm, the small fraction of Cu is difficult to be identified by TEM in Fig. 1(d), which means that the Cu2O phase is dominant in this film.
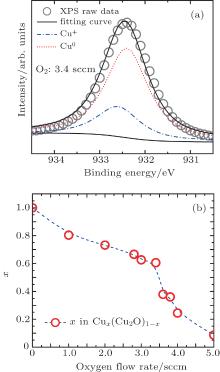
To further demonstrate the component change of these granular films and acquire the x value quantitatively in CuxCu2O1− x, the XPS data was used to calculate the atomic ratio of Cu. Figure 2(a) is the typical XPS data of the sample prepared under an oxygen flow rate of 3.4 sccm. The corresponding Gaussian fitting of metallic Cu and Cu+ peaks is marked by the dotted line and the dash-dotted line, respectively. The atomic ratio of Cu x in CuxCu2O1− x granular films can be calculated from the ratio of Cu peak area to total area. As shown in Fig. 2(b), x drops from 1.00 with the oxygen flow rate 0.0 sccm to 0.09 with the oxygen flow rate 5.0 sccm. The reduction of x is consistent with the character of TEM images in Fig. 1.
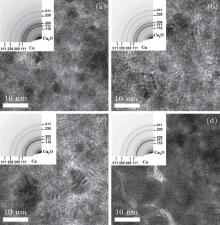 | Fig. 1. TEM images of the granular films sputtered in the different oxygen flow rates at (a) 2.0 sccm, (b) 3.0 sccm, (c) 3.4 sccm, and (d) 4.0 sccm, respectively. |
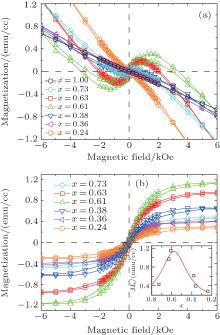
Figure 3 shows the magnetization (M– H) curves, where the raw data of the samples for different x is shown in Fig. 3(a). The M– H curve with x = 1.00 corresponds to the pure Cu sample. The linear diamagnetism signal of the pure Cu indicates that the samples are not contaminated by any magnetic impurities. After subtracting the diamagnetic background, the M– H curves are shown in Fig. 3(b), in which all of the samples possess room-temperature FM and the largest saturated magnetization is about 1.15 emu/cc at x = 0.61. As an estimate, the saturated magnetization is about 0.147 emu/g in Cu0.61(Cu2O)0.39 granular film without intentional magnetic doping, which is comparable to that in Cu2O fine powder[25] and that in a transition metal doped Cu2O system.[27] It is noticed that with the gradually decreasing x, the saturated magnetization first increases and then decreases, as shown in the inset of Fig. 3(b). This phenomenon may be understood by the change of x and the amount of defects in granular films, which will be addressed in the following text.
It is known that the FM is correlated with the defects in ODMS without transition metal impurities. To obtain the locations of defect levels in the band gap, the absorption spectra and the PL spectra are performed. The laser wavelength of excitation light in the PL measurement is 350 nm. In Fig. 4(a), α and hν represent the absorption coefficient and the energy of the incident photon. Compared with the band gap of pristine Cu2O (2.17 eV), all of the samples have shown enlarged band gaps from 2.78 eV to 3.04 eV. This blue shift is induced by the quantum confinement effect of Cu2O nano-granular films. Since the band gap is enlarged as the nanoscale particle size reduces, the Cu2O particle size in our granular films possibly becomes smaller with the increasing oxygen flow rate. Nonetheless, the maximum difference of the band gaps is only 0.26 eV within all fitting curves in Fig. 4(a), implying a minor variation of the particle size in these films. According to the relationship between the band gap and the Cu2O particle size, [28] the estimated Cu2O particle size is around 2.0 nm in all of the samples. Figures 4(b)– 4(d) exhibit the PL spectra with the Gaussian fitting curves for samples at x = 0.63, 0.61, and 0.24, respectively. The main peak with the blue dashed line around 2.9 eV results from the near band-edge transition. The locations of the main peaks in Figs. 4(b)– 4(d) are in accordance with the optical band gap calculated from absorption spectra in Fig. 4(a). Since the Cu vacancies in Cu2O introduce an acceptor level about 0.4 eV above the valence band (VB) edge, the green peaks marked by dash-dotted line at 2.4– 2.5 eV can be attributed to the electron transition from the conduction band (CB) edge to the Cu vacancies impurity level.[29, 30] Besides, the peaks with an energy higher than 3.0 eV are associated with the transition from the sub-levels in the CB to the VB. These sub-levels are contributed by Cu 4p states, which have a close relationship with the interaction between neighboring Cu atoms.[31]
As discussed above, the Cu vacancies are related with the low energy peaks (green) in Figs. 4(b)– 4(d). The ratio, the area of the green peak divided by the total area of the PL spectra, is 42.7%, 45.4%, 29.3% for x = 0.63, 0.61, and 0.24, respectively. The variation tendency of the ratio for Cu vacancies is similar to the M– x curve in the inset of Fig. 3(b). Thus, the FM can mainly be attributed to the Cu vacancies. However, this does not mean that the magnetization linearly varies with the amount of Cu vacancies. For instance, the variation of magnetization is pronounced even though the minor difference in the Cu vacancy ratio between x = 0.63 and x = 0.61 samples, which probably arises from the different distributions and different contact areas of Cu and Cu2O phases in the granular films. Actually, the FM observed in the granular films could be explained by the charge transfer ferromagnetism model proposed by Coey et al.[32, 33] When the defect states exist near the Fermi level, the Stoner criterion for spontaneous FM is satisfied because the Fermi level is located at a peak within the defect states. Due to the different work functions of Cu (4.3– 4.6 eV) and Cu2O (4.8 eV), [34– 36] the electrons may transfer from Cu towards Cu2O. As a result of the charge transfer, the Fermi level may shift to a new position, probably pinned at the defect states introduced by Cu vacancies. The work function difference 0.4 eV coincides with the relative location of the Cu vacancy energy level based on the first principles calculation.[37, 38] Consequently, the Fermi level is pinned at defect states and the spontaneous FM emerges. In our experiments, the FM can be modulated by the Cu/Cu2O interface in granular films. When x decreases from 1.00 to around xc (0.61) with an increased O2 flow rate, the volume ratio of Cu2O gradually increases. The interface area between Cu and Cu2O grows, leading to a higher density of states value at the Fermi level and an increased saturated magnetization due to a large amount of charge transfer. Below xc is the second stage. Although the volume ratio of Cu2O keeps increasing, the contact interface area may reduce substantially since there is insufficient metal Cu to cover the surface of Cu2O. Thus, the amount of the charge transfer shrinks, resulting in the decrease of the saturated magnetization, as shown in the inset of Fig. 3(b).
CuxCu2O1− x (0.09 ⩽ x ⩽ 1.00) granular films were prepared by reactive DC magnetron sputtering with different oxygen flow rates. The XPS data and TEM images prove that the films are composed of Cu and Cu2O. The Cu vacancies in all of the samples are crucial in providing local defect states near the Fermi level for the charge transfer induced FM. By tuning the ratio of Cu/Cu2O and their contact interface area, a vital route to control the magnetization is achieved. The room-temperature FM is observed in all of the films and the largest value of the saturated magnetization is 1.15 emu/cc. These results may offer further our understanding of the FM in undoped ODMS and they offer a relatively easy method to control the magnetization.
Wang Wei-Hua would like to thank Dr. Gong Cheng at UT Dallas and Dr. Xu Jian-Ping at Tianjin University of Technology for the fruitful discussion.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|