†Corresponding author. E-mail: zhang jian@jlu.edu.cn
‡Corresponding author. E-mail: ydp@jlu.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 50772043, 51172087, and 11074089).
Uniform InN nanowires were studied under pressures up to 35.5 GPa by using in situ synchrotron radiation x-ray diffraction technique at room temperature. An anomalous phase transition behavior has been discovered. Contrary to the results in the literature, which indicated that InN undergoes a fully reversible phase transition from the wurtzite structure to the rocksalt type structure, the InN nanowires in this study unusually showed a partially irreversible phase transition. The released sample contained the metastable rocksalt phase as well as the starting wurtzite one. The experimental findings of this study also reveal the potentiality of high pressure techniques to synthesize InN nanomaterials with the metastable rocksalt type structure, in addition to the generally obtained zincblende type one.
Semiconductors of III– V groups, especially the nitrides, have become a class of extremely important technological materials over the past decade. Their large direct band gap and high thermal conductivity, as well as high hardness, make them suitable for the fabrication of optoelectronic and electronic devices.[1– 5] In particular, indium nitride (InN) has received substantial attention since it has a direct band gap, the smallest electron effective mass, the largest mobility, the highest peak and saturation electron drift velocities among all the III-nitride compounds.[6, 7] The structures and properties of InN under various temperature– pressure conditions have been the focus of intense research efforts.
InN crystallizes in a hexagonal wurtzite structure (space group (SG) P63mc, No. 186, Z = 2) at the ambient condition.[8, 9] Two cubic phases, namely, the rocksalt-type structure (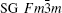
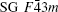
It is widely reported that the compression behaviors of bulk crystals and their nanoscale counterparts show differences to a large extent. A relevant example is GaN, the phase transition of which becomes irreversible when the size of the crystallites reduces to the nanoscale.[20] In this paper, we would like to report the compression behaviors of uniform InN nanowires under high pressures. An unusually irreversible transformation is found to be associated with the downstroke evolution.
The samples of InN nanowires were prepared by a CVD method.[21] 0.1 g high purity (99.99%) In powder, which served as the starting material, was placed uniformly in a quartz boat. The quartz boat was loaded at the center of a quartz tube, which was placed in a horizontal tube furnace. The quartz tube was degassed and then purged with high purity (99.999%) nitrogen several times. High purity (99.999%) ammonia was employed as the reactive nitrogen source. During the growth, the flow rate of ammonia was set at 400 sccm and the furnace was maintained at 740 ° C for 3 h. Finally, the tube and its contents were rapidly cooled down to room temperature with a cooling rate of 10 ° C/min under the protective flow of nitrogen. Fluffy products were collected from the downstream side of the starting materials in the quartz boat. A HITACHI S4800 field emission scanning electron microscope (FESEM) working at 25.0 kV was used to characterize the morphology of the as-prepared products. The transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) micrographs and the selected area electron diffraction (SAED) patterns were taken to study the morphology and the structure of the samples via a JEM-2200FS transmission electron microscope with an accelerating voltage of 200 kV, which was equipped with an energy dispersive spectrometer (EDS).
High-pressure experiments were conducted by using a symmetric diamond anvil cell (DAC) furnished with 400 μ m culet diamonds. The T301 stainless steel gasket was preindented by the diamonds to an initial thickness of about 53 μ m and then a center hole of 200 μ m in diameter was drilled as the sample chamber. The sample with the liquid quasihydrostatic pressure-transmitting medium (methanol/ethanol = 4:1) was loaded into the sample chamber along with a tiny ruby chip for pressure measurements. The angle dispersive x-ray diffraction (ADXD) experiments were carried out with the wavelength of 0.6199 Å at Beijing Synchrotron Radiation Facility (BSRF, Beijing, China). The ADXD patterns were collected using a Mar3450 CCD detector and the one-dimensional diffraction profiles of intensity as a function of 2θ were obtained by integration of the observed two-dimensional patterns with the Fit2D software.[22] The lattice parameters of the samples were obtained with Rietveld refinements of the powder x-ray diffraction (XRD) patterns performed using GSAS program packages.[23]
Figure 1(a) shows the SEM image of the as-prepared InN nanowires. The average diameter of these nanowires is estimated to be 86.7 nm (Fig. 1(b)). The typical TEM and HRTEM images and the corresponding SAED pattern of an isolated nanowire are shown in Figs. 1(c) and 1(d), which indicate that the growth orientation of these nanowires is along the [001] crystalline direction.
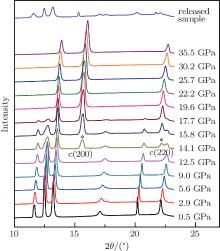
The in situ high-pressure ADXD patterns of the InN nanowires at various pressures up to 35.5 GPa are shown in Fig. 2. It can be seen that all the diffraction peaks shift to higher angles with increasing pressure due to the reduction of the unit cell volume. All the powder XRD patterns on the upstroke and of the released sample can be unambiguously indexed to the known phases of InN.
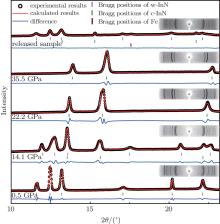
At 0.5 GPa, all the diffraction peaks can be indexed to a wurtzite-type phase (evidenced by the Rietveld refinement of the XRD pattern with Rwp = 4.15% shown in Fig. 3). The relatively stronger (002) peak also verifies that the preferred growth orientation of the nanowires is along the [001] direction, which is in good agreement with the HRTEM and the corresponding SEAD studies shown in Fig. 1. As the pressure is elevated, a dramatic change can be observed at 14.1 GPa. Two new peaks (marked with asterisks in Fig. 2) emerge in the diffraction patterns, indicating the onset of a high pressure phase transition. The new peaks can be assigned to (200) and (220) reflections of a rocksalt-type structure (evidenced by the Rietveld refinement of the XRD pattern at 14.1 GPa with Rwp = 3.83% in Fig. 3). It should be noted that the transition pressure (12.5– 14.1 GPa) is slightly higher than the experimental values (∼ 12 GPa)[9, 24] and the calculated ones (about 9– 12 GPa)[25– 27] of InN bulk materials. It can be rationalized by the general nano-size effects. The nanoscale materials, such as nanowires, have considerable surface areas and thus store much higher surface energy than their bulk counterparts. From this point of view, the InN nanowires need a higher pressure than their bulk counterparts to overcome the extra surface energy and to trigger the phase transition. Our results agree well with the report of Yao et al., who also observed the overshoot of transition pressure in InN nanomaterials.[14] As the pressure is further increased, all the peaks of the wurtzite-type phase become weaker. Up to 22.2 GPa, all the peaks of the wurtzite-type phase disappear and three new peaks appear instead, indicating that the phase transition is completed. The XRD pattern of the new phase fully corresponds to a rocksalt-type structure (evidenced by the Rietveld refinement of the XRD pattern at 22.2 GPa with Rwp = 5.54% in Fig. 3). No other structural transition is found until the pressure reaches the maximum value of 35.5 GPa in our experiment. This is also in good agreement with the previous reports in the literature.[9, 14, 17]
A significant finding in this study is that the phase transition of the InN nanowires is partially irreversible. When the samples were released to the ambient condition, as shown in Figs. 2 and 3, the rocksalt-type structure still remained coexistent with the starting wurtzite-type structure. The lattice parameters and atomic positions obtained from the refinements of the XRD data for the released sample are shown in Table 1. It is clear that all the parameters agree well with the previous work.[26] In the literature, almost all of the experimental results suggest that InN undergoes a fully reversible phase transition, with a clear hysteresis effect observed in the downstroke.[9, 14, 17, 28] The phenomenon found in this study is similar to that of GaN under high pressures, which also shows irreversible behaviors when the size of the crystals reduces to the nanometer scale.[20] However, such a possible partially irreversible phase transition has never been reported for InN before. In addition, compared to that of bulk and nano InN in the previous reports, [9, 14] a relatively stronger (002) peak may be found in the XRD pattern of the released sample. It suggests that the preferred orientation along the [001] direction has been retained during the phase transition. This may induce a higher kinetic barrier so that a portion of the sample may retain the rocksalt-type structure. Other factors, such as the transformation rate, nonhydrostatic effects, etc., may also be involved in the coexistence of wurtzite- and rocksalt-type InN. Time-dependent effects of the phase transformation, including aging of the quenched sample, also need further investigation.
| Table 1. Lattice parameters and atomic positions obtained from refinements of the XRD data for the released sample. |
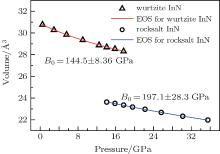
We also studied the volume evolution of the InN nanowires as a function of pressure, as shown in Fig. 4. The dependence of the unit cell volume on pressure is fitted to the third-order Birch– Murnaghan equation of state. For the wurtzite phase, the volume exhibits a monotonous decrease with increasing pressure up to 14.1 GPa. Then, there is a drastic reduction in the unit cell volume at 14.1 GPa accompanied by the phase transition from the wurtzite structure to the rocksalt structure. The relative volume reduction at the transition point is close to 16.37%. The fitting also yields that the bulk moduli of the wurtzite phase and the rocksalt phase are B0 = 144.5 ± 8.3 GPa with 

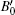

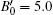
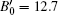
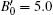
We have investigated the compression behaviors of one-dimensional nanostructured InN by using in situ x-ray diffraction techniques under high pressures. A wurtzite to rocksalt phase transformation was observed to initiate at 12.5– 14.1 GPa and complete at 19.6– 22.2 GPa in the upstroke of pressure. No other structural change was found until the pressure reached the maximum value of 35.5 GPa in our experiment. However, an anomalous phenomenon occurred when the sample was released to the ambient condition. The rocksalt-type structure retained and coexisted with the starting wurtzite-type structure. Such a possible partially irreversible phase transition is quite different from the previously reported results about InN, which indicated that the wurtzite to rocksalt phase transformation should be completely reversible. These abnormal behaviors of InN nanowires under high pressures might be attributed to the nano-size effect and the morphology-dependent thermodynamic and kinetic factors. The results of this study strongly indicate that high pressure techniques may be applicable for the fabrication of metastable InN nanomaterials.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|