†Corresponding author. E-mail: dwhe@bjtu.edu.cn
‡Corresponding author. E-mail: yshwang@bjtu.edu.cn
*Project supported by the National Basic Research Program of China (Grant Nos. 2011CB932700 and 2011CB932703), the National Natural Science Foundation of China (Grant Nos. 61335006, 61378073, and 61077044), and the Beijing Natural Science Fund (Grant No. 4132031).
A composite of graphene/PANI/GAunano is synthesized using the co-blend method. The morphologies and microstructures of samples are examined by transition electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR). Moreover, the microwave absorption properties of both graphene/PANI and GO/PANI/ GAunano composites are investigated in a microwave frequency band from 1 GHz to 18 GHz. The maximum reflection loss (RL) of GO/PANI/GAunano with a thickness of 2 mm is up to −24.61 dB at 15.45 GHz, and the bandwidth corresponding to RL at −10 dB can reach 4.08 GHz (from 13.92 GHz to 18.00 GHz) for a 2-mm-thick layer. The electromagnetic data demonstrate that GO/PANI/GAunano can be used as an attractive candidate for microwave absorbers.
The microwave absorption technique is a key issue of modern research because of the requirement to reduce pollution to the environment and subsequent damage to human beings. Microwave absorption is a process in which the wave cannot permeate through or be reflected by a material.[1] The absorption of the material depends on the frequency complex permittivity, complex permeability, and layer thickness.[2] Magnetic losses and dielectric losses are two important electromagnetic properties of microwave absorbers. There are two ways to enhance the absorption of incident waves: one is to enhance magnetic losses and the other is to improve dielectric losses.[3] Polyaniline (PANI) is a promising material for microwave absorption applications because it is inexpensive, environmentally stable, easy to process, and it exhibits excellent electrical conductivity.[4, 5] However, a single polymer is not enough to produce larger electric or magnetic losses. Therefore, PANI-based composites, such as carbon nanotubes, carbon fibers, and graphene have been popularly synthesized to further develop the electrical conductivity.[6, 7] Due to the high surface area, excellent conductivity, and remarkable mechanical stiffness, graphene is an outstanding carbon material that is used to form a composite with PANI.[8, 9]
Mie theory (the classical electromagnetic theory of spherical particles) was developed nearly a century ago and it plays a crucial role in describing the optical properties of metal nanoparticles, especially silver and gold particles.[10] Metallic nanoparticles exhibit strong and tunable surface plasmon resonance (SPR) when interacting with a microwave.[11, 12] In small metallic nanoparticles, localized surface plasmon polaritons can be excited at discrete frequencies.[13] This was experimentally demonstrated by Ritchie.[14] Due to their unique optical and electrical properties, metallic nanoparticles can be used in biosensors, [15] surface-enhanced Raman spectroscopy (SERS), [16] and microwave absorption.[17, 18] Peng et al. synthesized a core– shell structure by silver nanoparticles coated with Ni0.5Zn0.5Fe2O4 spinel ferrites, which exhibit good reflection losses (− 32.871 dB).[2] Melvin et al. grafted silver nanoparticles onto the surface of carbon nanotubes, which also have good reflection loss (− 21.9 dB).[19] Basavaraja et al. reported a polyaniline– gold/graphene oxide composite, whose electromagnetic interference shielding effectiveness is enhanced.[18] However, the studies of gold nanoparticles for the applications in microwave absorbers are very limited.
In this research, we synthesize GO/PANI/GAunano (GPAunano) nanocomposite by using graphene oxide (GO) as a substrate to adsorb aniline monomer. GO/PANI/GAunano has a great improvement in relative complex permittivity and permeability compared with the GO/PANI nanocomposite. In our work, the maximum reflection loss of GPAunano is up to − 24.61 dB at 15.45 GHz, and the bandwidth corresponding to RL at − 10 dB can reach 4.08 GHz (from 13.92 GHz to 18.00 GHz) for a 2-mm-thick-layer.
All of the chemical reagents used in this study were analytical reagents.
Graphite powder, concentrated sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), aniline, tetrachloroauric acid (HAuCl4), sodium citrate, sodium silicate, hydrochloric acid (HCl), were purchased from Beijing chemical factory and directly used without further purification. The deionized water was treated by an Aquapro ED12-3002-U system. Aniline was distilled under the protection of high purity N2 before being kept in a refrigerator. GO was synthesized using natural graphite powder by the modified Hummers method.[20]
The purified GO (0.189 g) was added into 50-mlr DI H2O, and the solution was ultrasonicated for 60 min to let the GO fully dispersed. Then, 52.5-ml HCl (12 mM), 1.955-g aniline monomer, and 50-ml ethanol were added into the GO solution and stirred for 1 h at − 10 ° C to form a uniform mixture. The oxidant, 3.1948 g (NH4)2S2O8 (APS), was dissolved in 17.5-ml HCl (12 mM) and the mixture was kept in a fridge. The polymerization was performed by rapidly adding the precooled oxidant solution, and the mixture was stirred for 24 h at − 10 ° C. Emerald flocculent precipitates were obtained and washed with a large amount of HCl (1 mM), ethanol, and DI water. The resulting precipitates were diluted into 100-ml DI water.
The Au nanoparticles were prepared according to the sodium citrate reduction method of HAuCl4 in GO solution.[21] GO powder dispersed in 50-ml DI water was subjected to ultrasonication for 60 min to give a stable suspension of GO and then centrifuged at 3000 rpm for 30 min to remove any aggregates remained in the transparent light brown exfoliated GO suspension. Then, 200-µ l HAuCl4 (0.01 mM) were added into the above solution, and the solution was mixed with vigorous magnetic stirring. The mixture was heated to 90 ° C followed by the addition of 1-mL sodium citrate (0.1 mM). The solution was heated for 30 min. The resulting GAunano suspension was put into dialysis for two days.
The 50-ml GO/PANI solution and 50-ml GO-Aunano solution were blended and ultrasonicated for 5 h.
The samples were characterized by TEM (JEM-1400 operating at 120 kV), FTIR (Thermo Fisher Nicolet 6700), and XRD (BRUKER) equipped with Cu Kα radiation (k = 0.15406 nm). The electromagnetic (EM) parameters of the composite were measured by an HP8722ES network analyzer.
Figure 1 shows the TEM images of GO, GP, and GAunano nanocomposites. Figure 1(a) is the TEM image of pure GO sheet. After composite reaction, aniline monomer is adsorbed on the surface of GO due to the electrostatic attraction, as shown in Fig. 1(b) and the inset image with high resolution. Fig. 1(c) shows that gold nanoparticles are adsorbed on the surface of graphene, which prevents their particles from being agglomerated. It can be seen in Fig. 1(d) that the gold nanoparticles are uniformly distributed on the composite. In addition, GO sheets are covered by PANI arrays evenly, indicating that the nucleation and growth processes occur only on the surface of the GO sheet.
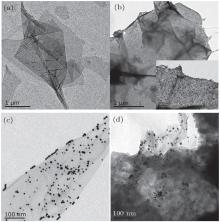 | Fig. 1. TEM images of GO (a), GP (b), inset image with a different resolution, GAunano (c), GPAunano (d) nanocomposites. |
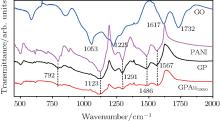
Figure 2 shows the FTIR spectra of GO, PANI, GP, and GPAunano nanocomposites. In the FTIR spectrum of GO, absorption peaks at 1732 cm− 1, 1617 cm− 1, 1225 cm− 1, and 1053 cm− 1 correspond to C= O characteristic absorption of − COOH, O– H flexural vibrations attributable to the vibrations of the residual water, C– OH stretching vibration and C– O stretching vibration, respectively.[22] In the spectrum of GP shown in Fig. 2, absorption peaks centering at 792 cm− 1, 1123 cm− 1, 1291 cm− 1, 1486 cm− 1, and 1557 cm− 1 are attributed to the flexural vibrations inside and outside aromatic C– H plane, C– N in PANI, the aromatic C= C stretching vibration, and the vibration of C= N respectively, [23] among which 1123 cm− 1 is the characteristic peak of conductive PANI. The characteristic peak of GO at 1732 cm− 1 and the disappearance of 1617 cm− 1 prove the polymerization of aniline monomers by the adsorption on the surface of GO and the participation of the surface functional groups of GO in the polymerization process. It is possible that − COOH on the surface of GO, as a kind of proton doping agent, could be connected to N atoms in PANI.
Figure 3 shows the XRD patterns of GO, GP, GPAu nano nanocomposites. The XRD pattern of the GO shows a sharp peak at 2θ = 10.54° , corresponding to the (001) reflection of GO.[22] In the cases of GP and GPAunano, diffraction peaks corresponding to both GP and Au can clearly be seen. The diffraction peaks at 9.41° , 14.91° , 20.72° , 25.55° are derived from PANI and those at 38.32° , 45.58° , 64.76° , 77.81° come from Au, [24] while the characteristic diffraction peak of GO disappears.
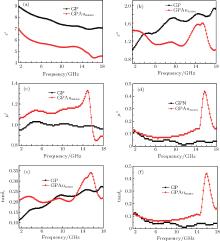
The microwave absorption property of material is generally determined by the complex relative permittivity and permeability. Figure 4 shows the values of complex relative permittivity real part (ε ′ ) (a), relative permittivity imaginary part (ε ′ ) (b), relative permeability real part (μ ′ ) (c), relative permeability imaginary part (μ ′ ) (d), dielectric loss tangent (tanδ ε = ε ′ / ε ′ ) (e), and magnetic loss tangent (tanδ μ = μ ′ / μ ′ ) (f), for GP and GPAunano. From Figs. 4(a) and 4(b), it can be seen that both ε ′ and ε ′ values of GP are higher than those of GPAunano in a range of 4 GHz– 18 GHz. But, ε ′ of GPAunano has a minimal peak at 16.3 GHz and ε ′ of GPAunano has a maximal peak at 15.45 GHz. The μ ′ of GPAunano has a maximal peak at 15.62 GHz as shown in Fig. 4(d). This shows that a resonance absorption is produced due to the introduction of gold nanoparticles.[12] When gold nanoparticles absorb microwaves, a closely induced current will be produced inside the material, which is in accordance with the work of Peng et al.[2] An electromagnetic field is then formed around the gold nanoparticles before the increasing electromagnetic loss. We can also see that the values of tanδ μ are higher than the values of tanδ ε are nearly at a frequency of 15.45 GHz. This indicates that the magnetic loss plays a main role in the microwave absorption nearly at the frequency of 15.45 GHz.
To further study the microwave absorption properties, the reflection loss (RL) is used to represent the microwave absorbing properties. The RL values of GP and GPAunano are calculated as follows:
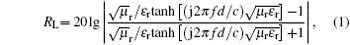

In the above equations ε r and μ r are the relative complex permittivity and permeability, respectively; f is the frequency of microwave; d is the layer thickness; and, c is the velocity of microwave in free space.
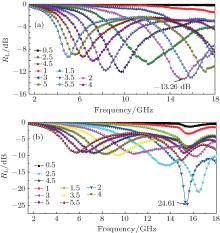
In Fig. 5(a), it can be observed that the maximum RL of GP is − 13.26 dB at 14.94 GHz and the bandwidth corresponding to RL at − 10 dB can reach 2.72 GHz (from 13.92 GHz to 16.64 GHz) for a layer of 2 mm in thickness. But, for GPAunano (Fig. 5(b)), the maximum RL value is − 24.61 dB at 15.45 GHz and the bandwidth corresponding to RL at − 10 dB can reach 4.08 GHz (from 13.92 GHz to 18.00 GHz) for a layer of 2 mm in thickness. The GPAunano composite exhibit an excellent microwave absorption performance in comparison with GP (Fig. 5(a)), which is due to the microwave-induced resonance effect produced by gold nanoparticles that increases the microwave absorption and reduces the reflection losses.
The excellent microwave absorption performance of the GO/PANI/ Aunano nanocomposite is attributed mainly to the following crucial factors: gold nanoparticles can absorb microwaves and a closely induced current will be produced inside the material, this generates an electromagnetic field, [2] thus increasing the electromagnetic loss. Ultimately, the microwave absorption capacity of the material is improved.
The ternary composites of GO/PANI/GAu nano are successfully prepared via a co-blend method. The obtained nanocomposite shows the enhanced microwave absorption properties compared with GO/PANI. The maximum RL of a 2-mm-thick GO/PANI/GAunano layer is up to − 24.61 dB at 15.45 GHz, which is more than − 13.26 dB at 14.94 GHz of GO/PANI. The bandwidth corresponding to RL of a 2-mm-thick GO/PANI/GAunano layer at − 10 dB can reach 4.08 GHz (from 13.92 GHz to 18.00 GHz). The introduction of gold nanoparticles significantly enhances the microwave absorption of the composite. This demonstrates that GO/PANI/GAunano can be a promising material with excellent microwave absorption properties.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|