†Corresponding author. E-mail: lohrasebi@nano.ipm.ac.ir
Kinesin is a microtubule-associated motor protein which can respond to the external electric field due to its polarity. Using a molecular dynamics simulation method, the effect of such a field on the affinity of kinesin to the α β-tubulin is investigated in this study. To consider kinesin affinity, the system is exposed to an electric field of 0.03 V/nm with frequency values of 1, 2, …, 9, and 10 GHz. It is found that the applied electric field can change kinesin affinity to the microtubule. These changes could perturb the normal operation of kinesin, such as the processive motility of kinesin on the microtubule.
Kinesin family proteins are stepping motor proteins which use the stored chemical energy of adenosine triphosphate (ATP) molecules to move along microtubule’ s filaments in the cells, and perform various tasks, such as intracellular cargo transport and cell division.[1, 2] Structurally, a kinesin is made up of a cargo-binding part, an α -helical stalk, and two identical globular motor heads. The cargo-binding part is composed of two light chains and is connected to the motor heads by means of an α -helical stalk. Each head is composed of a domain for binding the ATP molecule, and a domain for binding the head to the α β -dimer of the microtubule. The ATP binding domain can be in three states as follows: empty state, containing an ATP, and containing an adenosine diphosphate (ADP) molecule.[3– 6]
In each step of kinesin motion along the microtubule, the distance of 8 nm is passed through and the three mentioned states are present by hydrolyzing one ATP molecule into an ADP molecule and a phosphate group in the ATPase domains of the heads. It is worth mentioning that kinesin travels in a specific direction along a microtubule towards the plus end which, in most cells, brings about transporting cargo from the center of the cell towards the periphery.[7, 8] Since kinesins play vital roles in cells, they have been investigated in several aspects. For instance, in some experimental and molecular simulation studies it has been shown that the kinesin walks in a hand-over-hand fashion by coordinated motions of the two heads.[9– 11] Furthermore, the ATP hydrolysis cycle in the ATPase domain of the kinesin heads has been investigated in some other studies.[3– 5] On the other hand, the interaction between the kinesin heads and the α β -dimer of the microtubule has been considered.[12]
Since biological systems such as proteins have polar or charged groups, investigating the effects of external electromagnetic fields (EMFs), which arise from sources such as cell phones, on the operations of these biological systems seems important.[13– 22]
In this paper, the effect of an external electric field on the kinesin affinity to the microtubule is investigated in the presence of ATP and ADP and in the absence of such nucleotides. It is found that exposing the kinesin to the external GHz electric field changes the kinesin affinity to the microtubule. The changes in the properties of kinesin could perturb its biological function and in turn influence cell functions, which could have positive or negative biological consequences.
The initial structures of the ATP state of kinesin and α β -tubulin dimer are taken from the Protein Data Bank under the name of 4AQV.pdb.[3] The parameters of the GROMOS96-43a1 force field are used in all simulations. Then, the protein is placed in a cubic solvent box of size 17.39 nm × 9.14 nm × 12.31 nm in a way that α β -tubulin direction is parallel to the z axis of the box. The SPC model is employed to compute the interactions between 5.8 × 104 water molecules in the box. Thirty-seven Na+ ions are added to keep each system neutral. Each system is simulated by using MD-based GROMACS 4.5.3 software, [23] in time-steps of 0.002 ps. In all simulations, the cut-off radius for Van-der Waals and electrostatic interactions was set at 1.2 nm. The electrostatic interactions are computed via the application of the particle-mesh Ewald model. Each system is equilibrated for 1 ns within a constant-(NVT) ensemble, and then within a constant-(NPT) ensemble, respectively. Then, each system is heated from 250 K to 310 K during 5 ns.
To create the initial structure of the ADP state of the kinesin, the third phosphate of the ATP is removed from the ATP binding domain of the kinesin head. Then the same steps, like the ATP state of kinesin and α β -tubulin dimer, are performed for both ADP and the empty state systems. Then these systems (three different nucleotide states) are equilibrated for 5 ns at a pressure of 1 bar (1 bar = 105 Pa) by using the Parrinello– Rahman algorithm and the final equilibrated structures of the systems are used in all simulations in order to consider the kinesin affinity to the tubulin dimer in the three different nucleotide states of kinesin.
Following the equilibration phase, each system is simulated for a period of 500 ps, in the absence of an applied electric field, i.e., E = 0. To consider the influence of the application of oscillating field on MT, these systems were exposed to a periodic electric field E = E0 cos(ω t), where E0 is the electric field amplitude of 0.03 V/nm, which is proportional to membrane potential and the values of field strength similar to those in some experimental and computational studies are adopted.[14, 18– 21] The frequency of field, ω , is set separately at 1, 2, … , 9, and 10 GHz.
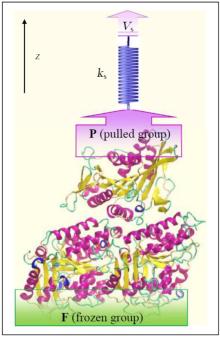
Investigation of interactions between kinesin and α β -tubulin is conducted by fixing some of the α β -tubulin atoms and pulling some of the kinesin’ s atoms along the z axis, mimicking AFM experiments, with the aid of a virtual spring.[19] For this purpose, two groups of atoms are defined, i.e., frozen (F) and pulled groups (P) as shown in Fig 1. In all simulations, the atoms of F group are fixed while the atoms of P group are attached to a virtual spring with an elastic constant of 1000 kJ/mol· nm2. The other end of the virtual spring is moved at a constant velocity of Vs = 0.01 nm/ps, along the defined z axis. In all of these three systems, kinesin is pulled till disconnecting from α β -tubulin, entirely. Figure 1 shows the computational system setup, schematically.
In this study, three simulation systems are designed to consider the effect of an applied external GHz electric field on the affinity of kinesin to the microtubule in the following three different conditions: containing an ATP (case 1), containing an ADP (case 2), and kinesin without nucleotide (case 3).
For each case, MD simulations are performed along the z direction and eleven groups of simulations are performed in the absence and presence of an electric field with various frequencies. During the simulations, the z coordination of the pulled group atoms, z(t), and the force which is experienced by the pulled group’ s atoms, F(t), are obtained based on the output data of the simulations. The F– z curve of each system is considered to experience a special force (SF) value at which the kinesin is detached from the microtubule. In Fig. 2(a), the F– z diagram of the system is shown in the absence of an electric field for case 1.
As it can be seen from this figure, the z value is gradually raised as the applied force is increased. The fluctuations in the protein elongation during its increasing are due to thermal motion of the protein atoms. An increasing trend can be seen in the F– z diagram till kinesin is detached from the α β -tubulin dimer, which occurs at the SF value, about 1560 pN, which is related to the kinesin affinity. After detaching kinesin from α β -tubulin dimer, the trend of the F– z curve changes in the declining or leveling off way. Figures 2(b)– 2(d) show the F– t, the z– t, and the Rg– t diagrams of case 1, where the Rg is the radius of gyration of the system. As can be seen in all of these figures at t ≈ 450 ps, the increasing trend of each curve changes, implying that kinesin is detached from the α β -tubulin dimer at t ≈ 450 ps.
The SF values for cases 2 and 3 are about 1140 pN and 1330 pN, respectively. It can be observed that the SF value in the absence of the electric field in case 1 is more than those in cases 2 and 3. Therefore, kinesin affinity to microtubule in the presence of ATP is biggest and in the presence of ADP is smallest, which is in good agreement with experimental results.
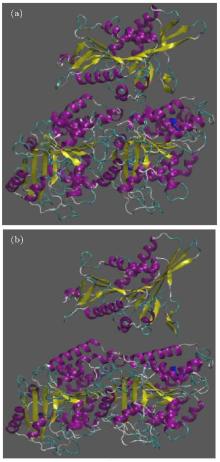
Due to observing the way kinesin is detached from the α β -tubulin dimer, two snapshots of the system are presented in Fig. 3. In this figure, panel (a) represents the initial structure of the protein, and panel (b) shows the protein after 500 ps in which kinesin has been detached from the tubulin dimer, for case 1.
As the maximal value (in the presence of ATP) and minimal value (in the presence of ADP) of the kinesin affinity play an important role in their functions, considering the external parameters which can affect these values seems necessary.
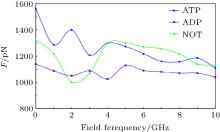
As mentioned above, since kinesin and α β -tubulin are polar proteins, they can respond to an external electric field. In this study, the external electric fields with different frequencies are applied separately to the system and the obtained results are shown in Fig. 4. As it can be observed, exposing the system to the external electric field causes the SF value to decrease in all cases. Applying an external alternating electric field can reduce the flexibility of the proteins, which leads to the reduction of the SF value. Therefore, these two proteins (kinesin and α β -tubulin) are separated from each other more rapidly. Reducing the flexibility of the proteins due to applying the GHz electric fields has been reported in our previous studies.[19– 21] Since the average time of the kinesin motion on the microtubule is related to the SF value, it is expected that exposing the system to the external GHz electric field leads to the decrease of this average time.
Furthermore, the minimal value of SF, corresponding to the presence of ADP in no electric field, is changed to a value in the case in which there is no nucleotide in the kinesin structure at 2-GHz frequency. By contrast, the maximal value of SF, corresponding to the presence of ATP in no electric field, is changed to a value in the case in which there is no nucleotide at frequencies of 4, 5, … , 8, and 10 GHz. Furthermore, the difference between max and min values of SF is reduced by increasing the frequency of the applied field. For instance, this difference value is 420 pN in the absence of an electric field, while this value is 90 pN at a frequency of 10 GHz. These effects could perturb kinesin’ s normal operation, such as the average time of the kinesin motion on the microtubule, which could affect the processive motility of kinesin on the microtubule.
In this paper, the effects of an external electric field on the kinesin affinity to α β -tubulin are investigated in three different conditions, i.e., kinesin containing ATP, containing ADP and without these nucleotides. It is observed that kinesin affinity to the microtubule in the presence of ATP is biggest and in the presence of ADP is smallest. Furthermore, in comparison with the E = 0 condition, applying an electric field with a frequency of 2 GHz causes the minimal value of SF to change to a value in the case in which there is no nucleotide in the kinesin structure and the maximal values of SF to a value in this case at frequencies of 4, 5, … , 8, and 10 GHz. Moreover, the difference between maximal and minimal values of SF is reduced by increasing the frequency of the applied electric field. Therefore, the obtained results show that applying an electric field could perturb kinesin affinity to the microtubule, which could have biological consequences such as disturbing kinesin processive motility.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|