†Corresponding author. E-mail: zafartariq2003@yahoo.com
The structural, electronic, and elastic properties of ZnSe1− xS x for the zinc blende structures have been studied by using the density functional theory. The calculations were performed using the plane wave pseudopotential method, as implemented in Quantum ESPRESSO. The exchange-correlation potential is treated with the local density approximation pz-LDA for these properties. Moreover, LDA+ U approximation is employed to treat the “d” orbital electrons properly. A comparative study of the band gap calculated within both LDA and LDA+ U schemes is presented. The analysis of results show considerable improvement in the calculation of band gap. The inclusion of compositional disorder increases the values of all elastic constants. In this study, it is found that elastic constants C11, C12, and C44 are mainly influenced by the compositional disorder. The obtained results are in good agreement with literature.
There is an increasing level of interest in II– VI wide band-gap (WBG) semiconductors because of their attractive technological applications, such as optical devices, [1] light-emitting and laser diodes, solar cells, and medical instrumentation.[2– 5] The main problems with these wide-gap semiconductor devices are the doping levels and the preparation of good ohmic contacts, especially for p-type materials. Most of the II– VI WBG semiconductors (e.g., ZnSe) are n-type or semi-insulating residuals, that can be doped n-type but cannot be doped p-type efficiently.[6, 7] ZnTe is the only II– VI WBG semiconductor presenting a p-type residual.[8] Indeed, the easiest way to artificially change the electronic and optical properties of II– VI semiconductors is by designing their alloys. To design these alloys, it is of interest to study ZnS, ZnSe, and ZnTe mixed in the ZnS1− xSex, ZnSxTe1− x, and ZnSexTe1− x ternary alloys in order to cover the full band spectrum. The gap bowing parameter of ZnSe1− xSx alloy is well known experimentally for all compositions.[9, 10] In addition, the optical properties of its epilayers have been studied using low-temperature photoluminescence (PL) and photo-reflectance spectroscopy.[11] For example, a ZnSe/ZnSe1− xSx hetero-structure is used in a blue semiconductor laser device with a high optical output and differential quantum efficiency at room temperature.[3] Several theoretical investigations have been carried out: (i) Ventkata et al.[12] studied a buffer on ZnS0.5Se0.5 based thin film solar cells, (ii) Kassali et al.[13] described composition and temperature dependent optical band gaps in ZnS1− xSex by using VCA and the empirical pseudo-potential method, (iii) Mesri et al.[14] reported a first-principles study of lattice constants and bowing parameter in ZnSe1− xSx compounds using zinc blende (ZB), CuAu-I, and chalcopyrite structures, (iv) ZnSe1− xSx films have been studied by Fridjine et al.[15]
In this paper, we made first principle calculations for the structural, electronic, mechanical, and elastic properties of sulfur-doped ZB ZnSe by local density approximation (LDA) plus optimized effective Hubbard parameter U (i.e., LDA+ U). The rest of this paper is arranged as follows. In Section 2, a brief description of the computational detail of this study is given. Explanations of the obtained results and discussion related to the structural, electronic, and elastic properties of S-doped ZB ZnSe compound are given in Section 3. The conclusions drawn from the calculated work are summarized in Section 4.
The well-established first-principles methods were used to study the structural, electronic, and elastic properties of materials. Theoretical calculations were performed by plane wave self-consistent field (PWSCF), which is a part of Quantum-ESPRESSO, [16] with LDA to exchange correlation functional given by Perdew and Zunger[17] and ultrasoft pseudo-potential to represent the interaction between ionic cores and valance electrons. Plane waves with a cutoff energy of 30 Ry were used to characterize the electronic wave functions, and Brillouin zone integrations were performed using 8 × 8 × 8 Monkhorst– Pack[18] grid of k-point mesh. The equilibrium lattice parameters and bulk moduli of ZnSe1− xSx were determined by the standard procedure for computing the total energy for different lattice constants and fitting to Murnaghan’ s equation of states. Since both LDA and GGA give very small energy gaps, a Hubbard U correction[19] was included with LDA to determine the electronic band gaps (i.e., calculations were performed with LDA+ U). Elastic constants were calculated by using the Lagrangian theory of elasticity.[20]
ZnSe and ZnS occur in ZB phase with space group F-43m. The cubic symmetry of ZnSe and ZnS is that the Zn atoms occupy the edges and face positions while the S and Se atoms at the inter-penetrating face occupy the centered cubic. In this way, the structure looks like a two-component diamond structure with no symmetry inversion. The alloys are modeled at some selected compositions x = 0.25, 0.5, and 0.75 with ordered structures. For x = 0.00, 0.25, 0.75, and 1.00, the structures are ZB but for x = 0.50, it is tetragonal with space group P-4m2 and has different lattice constants, a and c, as shown in the inset of Fig. 1. In these calculations, we performed an optimization of the lattice constants by energy minimization; i.e., different values of lattice constants were used to generate corresponding total energies at a cut-off energy value of 30 Ry. The optimized curves of ZnSe1− xSx alloys at x = 0.00, 0.25, 0.50, 0.75, and 1.00 are shown in Fig. 1 and their results are summarized in Table 1.
 | Fig. 1. Structural optimization plots for ZnSe1− xSx alloys at x = 0.00 (a), 0.25 (b), 0.50 (c), 0.75 (d), and 1.00 (e) versus lattice constant in ZB phase. |
| Table 1. Calculated lattice parameters (a and c) and bulk modulus (B) of ZnSe1− xSx alloys compared with experimental and other theoretical results. |
Usually, in the treatment of alloys, it is assumed that the atoms are located at the ideal lattice sites and the lattice constant varies linearly with composition x according to the so-called Vegard’ s law.[28] A careful analysis of the obtained results for these binary ZnS and ZnSe alloys shows that our results are in good agreement with other experimental and theoretical results.
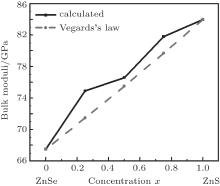
Figure 2 shows a comparison of calculated bulk moduli with Vegard’ s Law. The obtained energy and lattice constant data were fitted to the Murnaghan equation of state[29] in order to obtain the lattice constant and bulk moduli. The bulk moduli show a minor deviation from the Vegard’ s law. However, the deviation from Vegard’ s law has been reported in the literature for semiconductor alloys, both experimentally[30] and theoretically.[31– 34] The calculated bulk moduli (B) for ZnSe1− xSx are very close to the other reported results, as shown in Table 1. From Table 1, it can be seen that there is no significant difference between our calculated results and the experimental results. Figure 2 clearly indicates that the bulk modulus increases with the increase of S concentration in ZnSe and this causes the increase of its hardness.
The functional applications of materials in various electronic devices require a detailed understanding of energy band gaps along with aligned conduction band valleys. For the ZB phases, the electronic band structures of ZnSe1− xSx alloys were computed using LDA and LDA+ U. The band gap values obtained by LDA+ U for solids are in good agreement with the experimental data. The electronic band structures obtained with LDA+ U for the studied compounds, along high symmetry points in the first Brillouin zone from the calculated equilibrium lattice constants, are shown in Fig. 3. The band gap values computed by LDA and LDA+ U are compared in Table 2. In the ZB and tetragonal phases, both the valance band (VB) maximum and conduction band (CB) minimum reside at the Γ -point of the Brillouin zone for all of the considered dopant concentrations, showing that ZnSe1− xSx is a direct band gap material. From Table 2 it can be seen that the band gap values are higher in the case of LDA+ U, as compared with those obtained using LDA. The results obtained by LDA+ U show an improvement in the band structure compared with the underestimations commonly observed for LDA and GGA calculations. It has also predicted the band structures for ZnSe1− xSx along the high symmetry points in the first Brillouin zone from the calculated equilibrium lattice constants. Figure 3 shows the band structures for all of the selected compositions. All of these materials have direct band gaps along the Γ direction; the obtained results are listed in Table 2, together with other theoretical and experimental results. The band profiles and band gap values are in good agreement with the earlier theoretical results for ZnSe and ZnS.
 | Fig. 3. Band structures of ZnSe1− xSx at different concentrations of x: (a) x = 0, (b) x = 0.25, (c) x = 0.50, (d) x = 0.75, (e) x = 1.00. |
| Table 2. Calculated band gap of ZnSe1− xSx alloys compared with experimental and other theoretical results. |
The characters of the different bands become visible in the total and partial densities of states (DOSs) for the ternary compounds and are compared with the total DOS of all compounds, which are shown in Fig. 4. The bands below the Fermi energy (EF) are valence bands (VBs) and above they are conduction bands (CBs). In Fig. 4, the zero energy is taken at the Fermi energy level (EF level), in comparison with all of the binary compounds. The VB can be separated into three parts, where the lowest one (VB1) consists of a mixture of S 3s, Se 4s, and Zn 4s states whereas peak VB2 is due to Zn 3d states. The main valence band (VB3) is formed by p-states (S 3p and Se 4p). The conduction band (CB1) arises from the Zn 4s orbitals. It is commonly known that both LDA and GGA evaluate very small band gaps because this approximation locates the Zn 3d band incorrectly. This dilemma can be solved through LDA+ U approximation for the DFT calculations. From the DOS plots shown in Fig. 5, it is obvious that the band gap is increased for x = 0.00 to 1.00 in ZnSe1− xSx. With the LDA+ U method (U = 4.6 eV for ZnSe1− xSx at x = 0.00, 0.25, 0.75, 1.00, and 11.50 eV for x = 0.50), the obtained results are found to be more consistent with the experimental results, which prove that the increase in S concentration results in an increase in the energy gap, as shown in Fig. 5.
 | Fig. 4. Total and partial DOSs of ZnSe1− xSx at x = 0.00 (a), 0.25 (b), 0.50 (c), 0.75 (d), and 1.00 (e), each as a function of sulfur composition. |
The elastic constants are also important parameters to describe the mechanical properties of materials which undergo stress, and they provide significant information related to binding characteristic between adjacent atomic planes, structural stability, and anisotropic character of binding. There are 21 independent elastic constants, Cij, but the symmetry of cubic crystal reduces them to only three independent elastic constants: C11, C12, and C44. The elastic constants of solid materials also provide a link between the mechanical and dynamical behaviors of crystals, and they give useful information concerning the nature of the forces operating in solids. In particular, elastic constants provide information about the stability and stiffness of materials. Ab initio calculations of elastic constants require precise methods. Since the forces and the elastic constants are functions of the first-order and the second-order derivatives of the potentials, their calculations will provide a further check on the accuracy of the calculation of forces in solids. The second-order elastic constants (Cij) are calculated by energy minimization, and the results are listed in Table 3. For a stable tetragonal structure, there exists six independent elastic constants Cij (C11, C12, C13, C33, C44, and C66) which satisfy the well-known Born– Huang criteria for stability[41]C11 > 0, C33 > 0, C44 > 0, C66 > 0, (C11 − C12) > 0, (C11 + C33 − 2C13) > 0, [2 (C11 + C12) + C33 + 4C13] > 0. On the other hand, for the cubic structure, three independent elastic constants (C11, C12, and C44) satisfy (C11 − C12) > 0, C11 > 0, C44 > 0, (C11 + 2C12) > 0.[42] Our calculated results for elastic constants obey these stability conditions for each composition for considered ZnSe1− xSx compounds shown in Table 3. The results presented in Table 3 reveal that the elastic constants calculated in this study decrease with the increase in concentration of x. The values of C11, C12, and C44 are compared with other calculated results provided in Table 3. shear modulus (G) and Young’ s modulus (E) are measured for ZnSe1− xSx in order to determine their hardness, and both G and E are found to be decreased from higher to lower concentrations of x. The calculated Young’ s modulus E for ZnS has a higher value as compared with ZnSe and compounds of sulfur. Table 3 shows that ZnS has high hardness values in comparison to the others. The shear modulus G of materials represents the resistance to plastic deformation while the bulk modulus B is the resistance to fracture. According to the criterion of Shein et al., [43] a material becomes brittle if the B/G ratio is less than 1.75 and ductile for higher ratios. In the present case, the B/G ratio is high (nearly 2.14), as given in the Table 4 for the studied alloys, which indicates that the alloys will behave in a ductile manner.
| Table 3. Calculated elastic constants of ZnSe1− xSx alloys compared with experimental and other theoretical results. |
First-principle, based on the DFT with the LDA and LDA+ U, investigations of the structural, electronic, and elastic properties of ZnSe1− xSx alloy system are reported. Features such as lattice constants, bulk modulus, elastic constants, and band gap are determined. The composition dependent characteristics over the whole compositional range from pure ZnSe to ZnS are presented and discussed. Generally, our results are found to be in good agreement with the existing available data. For ZnSe1− xSx alloy system (x = 0.25, 0.50, and 0.75), these findings are predictions and they can be used as a reference for future work. Our conclusions can be drawn as follows. (I) The lattice constant of the alloy is found to be decreased, which is probably due to the size of the sulfur atoms. It is also found that the bulk modulus of the alloy is increased. (II) The calculated band gap of the alloy increases with the increase of sulfur concentration from 2.2448 eV to 3.528 eV in ZnSe. It is also observed that the band gap is wide and direct in the full range of the sulfur concentration. (III) The elastic constants C11, C12, C44, Young’ s Modulus, and shear modulus have increased from pure ZnSe to ZnS. Only at x = 0.5, is an abrupt increase in elastic constant observed. This may be attributed to the change of structure from ZB to tetragonal. (IV) In most cases, the studied features exhibited a linear behavior with x except at x = 0.5 for elastic constants. In the present case, the value of B/G is higher (nearly 2.14) than 1.75 for the studied alloys, so they will behave in a ductile manner. Our results may provide information for the compositional characterization of materials under load in applications. (V) The electronic band gap shows that well-organized diffusion of the two pure compounds ZnSe and ZnS in the whole compositional range of ZnSe1− xSx enhances the scenario of tuning the band-gap energy (ranging from 2.6024 eV (ZnSe) to 3.5280 eV (ZnS)) and the lattice constants of these compounds. On the other hand, it substantially increases the potential of ZnSe1− xSx materials to be used as a light emitters, wave guides, and confinement layers in laser diodes.
The authors highly appreciate Prof. Dr. M. A. Choudhary for the fruitful discussion and giving access to the Simulation facilities in the simulation laboratory at the Department of Physics, The Islamia University of Bahawalpur.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|
| 43 |
|
| 44 |
|
| 45 |
|
| 46 |
|
| 47 |
|