†Corresponding author. E-mail: 2007zhangxiaofei@163.com
*Project supported by the National Natural Science Foundation of China (Grant Nos. 11204283 and 11304298), the Natural Science Foundation of Zhejiang Province, China (Grant No. LQ13A040002), and the Scientific Research Project Funds of Talent Introduction of China Jiliang University (Grant No. 01101-000406).
We report the microstructure evolution of copper (Cu) nm-sized atomic islands on silicone oil surfaces in the ambient atmosphere. The origin of these nearly free sustaining atomic islands is explained by a three-stage growth model. The first stage is the nucleation and growth of atomic granules. Subsequently, the compact atomic islands grow by the aggregation of the atomic granules. Finally, they adhere to each other and form branched atomic islands. During the characteristic evolution, the atomic granules reconstruct and the average height of the atomic islands increases from 7.0±1.0 nm to 13.0±1.0 nm. The detailed evolution mechanism of the Cu atomic islands is presented.
As the physical characteristics of the films strongly depend on their initial growth, quantitative understanding of the nucleation and growth processes of nm-sized atomic islands (or aggregates) has attracted widespread attention.[1– 6] Considerable theoretical[7, 8] and experimental[9– 11] effort has been made to investigate the growth of atomic islands under various experimental conditions. The fundamental process during island growth is closely related to adatom diffusion, nucleation, aggregation, coalescence, etc. The transition from the initial stage of nucleation to growth and coalescence for Ag/Pt(111) was studied by means of scanning tunneling microscopy.[12] For the homogeneous crystal nucleation process in a soft-core colloid model, the role of the prestructured surface cloud in crystal nucleation was reported.[13] In addition, a study of the initial stages of Cu epitaxy on Ni(100) exhibited that the smallest stable island abruptly changes from a dimmer to a tetramer.[14] It should be noted that the nucleation and growth processes of the atomic islands on solid substrates are momentary, which may be difficult to observe and study.
The nucleation and growth processes of atomic islands are strongly dependent on the nature of the substrate. Several liquid substrates (such as silicone oil and ionic liquid) have been used to grow nearly free sustained atomic islands (i.e., compact and ramified islands). Due to the weak interaction between the atomic islands and liquid substrates, the morphologies and microstructures of the atomic islands on liquid substrates are very different from those on solid substrates. The aggregation behaviors of atomic islands on liquid surfaces have been studied.[15] It was noted that the atomic islands perform Brownian motion on the oil surface and the diffusion coefficient of the islands containing as many as 1010 atoms is of the order of 10− 11 cm2/s, which is much larger than 10− 17 cm2/s of the atomic islands with 102 atoms on Ag(100).[10] Reshaping of the branched islands has also been investigated in situ in vacuum by optical microscopy.[16] Moreover, the evolution characteristics (i.e., random rotation, separation, and aggregation) of the branched islands have been studied.[17] The experiments showed that as the morphology evolution from the ramified atomic islands to the compact and ring-shaped atomic islands occurs, the apparent Ag coverage of the total area decays gradually in the ambient atmosphere. According to the experimental results, a two-stage growth model was summarized by Ye et al.[18] Furthermore, atomic force microscopy (AFM) measurements showed that these atomic islands are composed of atomic granules of size 10 nm.[19] However, to the best of our knowledge, the growth and diffusion processes of the atomic islands in the ambient atmosphere, especially the nucleation, growth, and adhesion of the atomic granules on the silicone oil substrate, have so far not been studied systematically.
In this paper, we provide a simple strategy to systematically study the microstructure evolution of Cu nm-sized islands on silicone oil substrates in the ambient atmosphere. The interesting evolution behavior shows a characteristic aggregation process of the Cu atomic islands. The microstructures of the islands are measured and their aggregation mechanism is discussed in detail.
The Cu (99.999% purity) islands (or aggregates) were grown on commercial silicone oil (Dow Corning 705 diffusion pump fluid) substrates by thermal evaporation at room temperature (289± 3 K). Silicone oil with a vapor pressure below 10− 8 Pa was painted onto a frosted glass surface, which was fixed 145 mm under the filament (tungsten). The base pressure prior to the deposition was 3.5× 10− 4 Pa. The deposition flux f was 0.1 nm/s. The nominal film thickness h was 0.4 nm and 1.0 nm, which was determined by a quartz-crystal balance (ULVAC CRTM-3000) and calibrated by an atomic force microscope (AFM, Veeco DI3000).
After deposition, the samples were kept in the evaporation chamber (under vacuum conditions) for a time period Δ t and then removed from the chamber. It should be noted that Δ t = 13 min is the minimum duration for the samples in our vacuum system and the air filling process takes at most 2 min. An optical microscope (Leica DMLM) was used to take images (with a time interval of 5 s) of the Cu islands on the oil surfaces immediately in the ambient atmosphere. After that, the islands, for the samples of h = 0.4 nm at observation time t = 0 and 120 min, were transferred from the oil substrates to glass surfaces and washed carefully with acetone and ethanol. Then the AFM (Veeco DI3000) was used to measure the surface morphology of the Cu islands and a high-resolution transmission electron microscopy (HRTEM, JEM-2010) was used to characterize the crystal structure of the islands.
The interesting results are visualized in Fig. 1, where the consecutive morphology evolution of the Cu atomic islands during 0– 120 min is given. Initially (t ≤ 3 min), the Cu islands could not be obviously discerned on the oil surfaces by the optical microscope. Then, compact islands with width of the order of 102 nm grow gradually. Subsequently, the compact islands (see the image of 3 min) diffuse and aggregate on the oil surface. If two islands meet, they stick with each other and become a branched island (see the images of 30 min, 60 min, and 120 min). Finally, Cu branched islands with clear morphologies form. The experiment was repeated several times to obtain reliable results.
 | Fig. 1. Optical microscope images taken at t = 0– 120 min showing the morphology evolution of the Cu islands; f = 0.1 nm/s, h = 0.4 nm, and Δ t = 13 min. Each image has a size of 54.0 μ m× 54.0 μ m. |
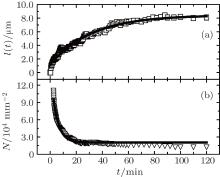
During the evolution of the Cu islands, the average gyration radius of the unified islands, l(t), gradually increases. The relation between l(t) and t is measured and shown in Fig. 2(a). The experimental data in Fig. 2(a) can be well fitted by a growth equation

where A0 and l(∞ ) are constants, and l(0) = − A0 + l(∞ ) and l(∞ ) represent the average gyration radii of the islands at t = 0 and t → ∞ , respectively. From Fig. 2(a), one can find that A0 = 7.3 μ m, l(∞ ) = 8.4 μ m, and the aggregation time constant τ = 30 min. For t ≤ 30 min, l(t) increases up to about 5.0 μ m, demonstrating that the Cu atoms and islands diffuse rapidly and rotate randomly on the oil surfaces in the ambient atmosphere. The aggregation of the Cu atoms and islands really occurs. For t > 30 min, l(t) increases slowly. On one hand, the diffusion of the large islands gradually becomes slow; on the other hand, the encounter of two islands is a rare event due to the decrease of the island number density N. Therefore, the increment of the islands, Δ l(t), decreases as t increases. In addition, the average width of the Cu branched islands (3 min ≤ t ≤ 120 min) remains nearly the same and equals 0.37± 0.06 μ m.
As t increases, the morphologies of the Cu islands become clear and the number density N of the Cu islands can be measured, which is shown in Fig. 2(b). It is found that N decreases rapidly with t. The experimental data in Fig. 2(b) can be well fitted by a decay equation
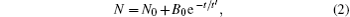
where N0 = 2.0 × 104 mm− 2 and B0 = 13.3 × 104 mm− 2 in this experiment, t′ ∼ 103 s is the average lifetime of the Cu islands, which is much larger than a few seconds on the solid substrates.[20] The experimental results may show the characteristic property of the silicone oil substrate, which is different from that on the solid substrates because of the pinning effect between the atoms and the substrates.[21] During the growth of the Cu islands, the morphology evolution may mirror the great changes in the microstructures.
In the present experiments, for h = 1.0 nm, the expeditious optical microscope measurements show that the Cu branched islands have formed. It is when h ≈ 0.4 nm that the evolution phenomenon can be observed. Besides, if Δ t was increased from 13 min to 180 min for the same sample, the evolution of the Cu islands remained nearly unchanged, indicating that the obvious aggregation of the Cu islands may not occur during the 180 min in the evaporation chamber. Why does the morphology of the Cu islands evolve gradually? We envision two possibilities: (i) the oxidization of the Cu islands and (ii) the perturbation of the rapid air filling process, which are discussed in the following section.
At the initial stage (t < 3 min), it is very difficult to discern the Cu islands clearly by the optical microscope and their morphology and microstructure are by far the most important and meaningful elements for characterizing their aggregation mechanism, therefore AFM measurements are greatly needed.
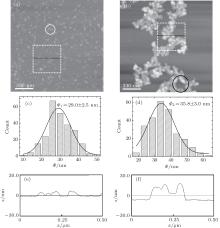
AFM measurements of the samples at the initial stage of growth and 120 min were performed. Typical AFM images of the Cu islands are shown in Fig. 3. The image in Fig. 3(a) shows many smaller dispersed Cu granules, and some of them have aggregated together (see the white circle), which are obviously different from the branched islands in Fig. 3(b). In Fig. 3(b), the branched islands are composed of a great number of smaller granules, which is basically in agreement with our previous results.[19, 22, 23] Figures 3(c) and 3(d) show that the corresponding average diameters Φ of the Cu granules are 29.0± 2.5 nm and 35.8± 3.0 nm, respectively. The average heights HAFM of the Cu islands at t = 0 and 120 min are 7.0± 1.0 nm and 13.0± 1.0 nm, respectively. The average width of the branched islands (in Fig. 3(b)) is 0.25± 0.07 μ m, which is about 5 times the granule diameter Φ and equals 0.37± 0.06 μ m in Fig. 1. The corresponding profiles along the lines of the Cu islands are shown in Figs. 3(e) and 3(f). It is found that HAFM < Φ ; in this case, the shape of the Cu granules may be regarded as an ellipsoid.
These experimental results reveal the following characteristics. (i) When two Cu granules meet, they irreversibly adhere to each other and diffuse as one unit. These units do not coalesce into a larger granule (see the white circle in Fig. 3(a)). (ii) The Cu granules in Fig. 3(a) grow gradually at the expense of Cu atoms or smaller size granules during the evolution. A large number of the Cu granules continue to diffuse and stick with each other on the silicone oil surfaces. Meanwhile, the granules squeeze each other and restructure. So the height of the islands increases and the total energy of the compact islands should decrease. (iii) The structure of the branched islands is not compact (see the black circle in Fig. 3(b)), which may involve the interconnections between the Cu islands and the silicone oil molecules.
The interesting experimental results suggest that the growth of the Cu islands should obey a three-stage growth model. Firstly, the deposited atoms diffuse and nucleate into a large number of Cu granules. Subsequently, the compact islands grow by the aggregation of the Cu granules, and finally they diffuse, collide with each other, and form branched islands. It should be noted that the two-stage growth model is the summary of the Cu island growth process observed in the optical photographs. In the present paper, the AFM images show the initial growth behavior of the Cu islands. Then the three-stage growth model is obtained. It is clear that the microstructures of the Cu islands do change remarkably during island growth. Furthermore, the internal microstructure of the Cu granules needs to be studied.
Figures 4(a) and 4(b) show the plain view high-resolution TEM micrographs of the samples at t = 0 and 120 min, respectively, in which the crystal grains (or crystallites) can be clearly observed, and the insets are the corresponding diffraction patterns. Figures 4(c) and 4(d) show the higher-resolution TEM images of the crystal grains in Figs. 4(a) and 4(b), respectively, in which the white arrows indicate different crystal plane orientations. By measuring the diameter a of the crystal grains, the diameter distributions are obtained. Figures 4(e) and 4(f) show that the average diameters of the crystal grains are 4.03± 0.60 nm and 4.24± 0.70 nm, respectively, which are much smaller than those of the Cu granules in Fig. 3. It can be inferred that (i) the Cu granules are composed of these crystal grains; and (ii) the possible oxidization of the Cu islands may not contribute to the morphology and microstructure evolution of the Cu islands.
With the above experimental results, we can further discuss the evolution of the Cu islands in Fig. 1. The average diameter of the Cu granules is about 33 nm, which is much smaller than the resolution of the optical microscope (about 200 nm). In this case, it is difficult to discern the islands clearly at the initial stage of the evolution. As the Cu granules diffuse and adhere to each other on the oil substrate, they squeeze each other and reconstruct; the growth of the Cu islands takes place. Finally, the size of the islands increases and their morphology gradually becomes clear. This evolution in the ambient atmosphere basically satisfies the three-stage growth model.
At the initial stage of the nucleation and growth of the Cu islands, the deposited Cu atoms are aggregated to a great number of monolayer Cu granules, which disperse evenly and diffuse speedily on the oil surfaces. As the single Cu granules meet, the Cu islands grow gradually. It is feasible to calculate the granule number density n at the initial stage of nucleation and growth of the Cu islands. From the optical image of t = 120 min, the apparent Cu coverage of the total area, namely, ρ , is 18.7± 1.0% and the average height is HAFM = 13.0± 1.0 nm. Therefore, the total Cu volume V per area on the oil surfaces can be obtained as V = ρ HAFM, which is a constant for Figs. 3(a) and 3(b). At the initial stage of the nucleation and growth of the Cu islands, the average height of the dispersed Cu granules is about 7.0± 1.0 nm (see Fig. 3(c)), so ρ = 34.0± 6.0% is obtained; and then 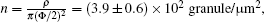
In order to minimize the surface free energy during the evolution of the Cu islands, the surface coverage ρ decreases and HAFM increases. Theoretically, we suggest that the geometrical shape of the Cu compact islands is similar to a disk.[18] In this nearly free standing system, the total free energy Gtotal can be given by
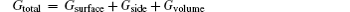
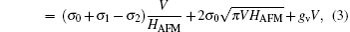
where Gsurface refers to the surface energy resulting from the upper and down surfaces of the islands, Gside is the lateral surface energy of the islands, and Gvolume is the volume energy stemming from the residual stress and stored within the islands. The HAFM can be read from the AFM images by means of AFM software, and V refers to the volume of the compact islands, which is a constant. The surface energy σ 0 and interface surface energy σ 1 are determined by the microstructure of the islands. The surface free energy of the silicone oil, σ 2, can be regarded as a constant. During the evolution, HAFM increases gradually. The island shape is determined by

The dependence of HAFM on σ 0, σ 1 can be obtained from Eq. (4)
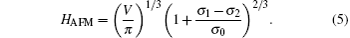
Equation (5) exhibits that both σ 0 and σ 1 change as HAFM increases during the growth of the islands. In other words, the microstructure of the islands evolves with t in the ambient atmosphere. In addition, the difference of the total free energy per area for the islands on the oil surfaces at t = 0 and 120 min, namely, Δ Gtotal, can be written as
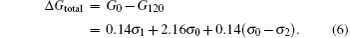
As σ 2 (3.60 × 10− 2 J/m2[25]) is much smaller than σ 0 (for Cu with the polycrystalline structure, σ 0 varies and its mean value is of the order of 1.79 J/m2[26]), σ 0– σ 2 > 0 is obtained, therefore, Δ Gtotal > 0, indicating that the total free energy of the islands decreases with t. Equations (3)– (6) give a theoretical explanation for the experimental results shown above.
We investigated the microstructure evolution of Cu islands grown on silicone oil surfaces in the ambient atmosphere, and the essential physical picture of the aggregation mechanism of the Cu islands is given. At the initial stage of the nucleation and growth of the Cu islands, nm-sized Cu granules grow and diffuse on the oil surfaces in the vacuum chamber. During the rapid air filling process, an air pressure gradient is introduced around the Cu atoms and granules. It provides energy for the Cu atoms and granules to diffuse speedily on the oil surfaces. As the Cu atoms and granules adhere to each other, the Cu islands grow gradually. In order to minimize Gtotal, the Cu granules in the Cu islands are reconstructed on the oil surfaces. The Cu granules collide and squeeze each other on the oil surfaces and some of them become higher. Therefore, HAFM increases gradually and then the surface morphology of the Cu islands becomes clearer. According to the experimental results, a three-stage growth model of the Cu islands is proposed.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|