†Corresponding author. E-mail: yqli@lnu.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant No. 11474141), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (Grant No. 2014-1685), the Scientific Research Foundation for the Doctor of Liaoning University, the Special Fund Based Research New Technology of Methanol Conversion and Coal Instead of Oil, and the China Postdoctoral Science Foundation (Grant No. 2014M550158).
Among many kinds of ways to study the properties of atom and molecule collision, the quasi-classical trajectory (QCT) method is an effective one to investigate the molecular reaction dynamics. QCT calculations have been carried out to investigate the stereodynamics of the reactions F + H2/HD/HT→FH + H/D/T, which proceed on the lowest-lying electronic states of the FH2 system based on the potential energy surface (PES) of the 12A’ FH2 ground state. Although the QCT method cannot describe all quantum effects in the process of the reaction, it has unique advantages when facing a three-atoms system or complicated polyatomic systems. Differential cross sections (DCSs) and three angle distribution functions P( θr), P( ϕr), P( θr, ϕr) on the PES at the collision of 2.74 kcal/mol have been investigated. The isotope effect becomes more obvious with the reagent molecule H2 turning into HD and HT. P( θr, ϕr), as the joint probability density function of both polar angles θr and ϕr, can reflect the properties of three-dimensional dynamic more intuitively.
The F + H2 reaction, as one of the most studied elementary chemical reactive processes, has been investigated widely both experimentally and theoretically. It remains a main prototype of an exothermic elementary chemical reaction, which has attracted a great deal of attention during the last four decades.[1– 27] Total and angular cross sections, rate constants, and product vibrational-rotational state populations are the primary quantities that have been probed for this reaction.[1] Chemical laser and chemiluminescence experiments reported that the product molecules have been made up with a strong population inversion since the 1960s.[2, 3] The landmark experiments of Lee and co-workers in the 1980s provided the vibrationally state-resolved differential cross sections (DCS) for F + H2 and their deuterated isotopic variants.[4, 5] During the 1990s, further molecular beams and photo-detachment experiments were added to the details.[6– 11] The experiments of Lee and co-workers revealed characteristic forward scattering peaks in some of the products of vibrational states that were attributed to scattering resonances, but the theoretical interpretation of these results proved to be challenging due to the lack of a precise potential energy surface (PES).[12] Fortunately, the appearance of the higher resolution measurements and new PESs breaks the challenge.[13– 19]
Many attempts have been adopted to extract the transition state based on employing increasingly larger basis sets and multireference configuration interaction (MRCI) method at variance with experimental data. Accurate crossed molecular beam measurements of the glory structure in the absolute integral cross sections for elastic F + D2 scattering have been reported by Aquilanti et al.[20] Afterwards, a new partly ab initio surface was constructed by the Truhlar group called 5SEC in 1991 based on the scaled externality correlation technique.[18] Manolopoulos and Werner constructed a fully ab initio PES of the F + H2 system called (SW), [21, 22] and there are enough remaining discrepancies for the F + H2 and F + D2 reactions to warrant a quantum mechanical investigation.[22, 23] The latter QCT was mainly used to study the reaction cross sections, rate constants, and scattering resonances for the F + H2 reaction and its isotope reactions.[24– 27]
In the present work, the QCT method has been adopted to study the stereodynamics of the F + H2/HD/HT→ FH + H/D/T reactions at the selected collision energy of 2.74 kcal/mol. The vibrational and rotational levels are taken as v = 0 and j = 0, respectively. The paper is organized as follows. In Section 2, we briefly describe the main details of the theoretical methodologies. In Section 3, the detailed results and discussion of the QCT simulations are accounted. Finally, the conclusions are summarized in Section 4.
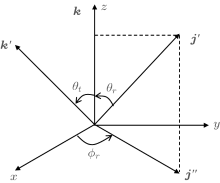
The angle relationship between the initial relative velocity and the final relative velocity vectors are described in the center-of-mass (CM) reference frame. As shown in Fig. 1, the z axis is parallel to the initial relative velocity vector k, and the y axis is perpendicular to the x– z plane containing the initial and final relative velocity vectors k and k′ . θ t is the angle between k and k′ (so-called scattering angle). θ r and ϕ r are the dihedral and the azimuthal angles of the final rotational angular momentum j′ . The distribution function P(θ r), which describes the k– k′ correlation, is expanded in a series of Legendre polynomials[28– 30]
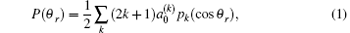
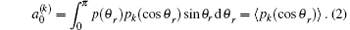
The coefficients 

Here, the average rotational alignment of the product is only calculated, because it has been measured in most experiments. P(θ r) is expanded up to k = 20, where it shows a good convergence. The dihedral angle distribution function P(ϕ r) describes k– k′ – j′ correlation. P(ϕ r) will be expanded in a Fourier series as

where

In the common calculations, P(ϕ r) is expanded up to n = 24 to acquire a good convergence. It should be noted that the DCS obtained here only describes the k– k′ correlation or the scattering direction of the product, which is not associated with the orientation or alignment of the product rotational angular momentum vector j′ .[31]
The QCT procedure has been carried out in many reaction systems up to now.[32– 42] In our calculations, the integration step is set at 0.1 fs to ensure a conservation of the results. In order to ensure no interaction between attacking atom F and the CM of the diatomic molecule H2/HD/HT, the distance between them is set to 15 Å . A branching of 100000 trajectories has been run for each of the reaction F + H2/HD/HT→ FH + H/D/T. The collision energy of 2.74 kcal/mol has been selected for our calculations with the reagent H2/HD/HT molecule being at the v = 0, j = 0 state. The orientation of the diatomic molecule and the phase of the diatomic vibrational motion were randomly sampled by a Monte Carlo procedure and the impact parameters are optimized before running the trajectories. Herein, the optimized impact parameters are 1.275 Å , 0.75 Å , and 0.73 Å for F + H2, F + HD, and F + HT reactions, respectively.
For the title system, our QCT calculations were performed on the 12A’ FH2 ground state PES constructed by Li et al., [43] which was based on the accurate single-sheeted double many-body expansion method. It was constructed based on the aug-cc-pVTZ and aug-cc-pVQZ basis sets with extrapolation of the electron correlation energy to the complete basis set limit, as well as the extrapolation to the complete basis set limit of the complete-active-space self-consistent field energy. The collinear and bending barrier heights of the new global potential energy surface is 2.301 and 1.768 kcal/mol, which is in very good accordance with the values of 2.222 and 1.770 kcal/mol (the best results of FH2 PES). Particularly, in the aspect of describing the important vander Waals interactions, the new potential energy surface can provide great contribution, which plays an important role in investigating the dynamics of the title system. That is to say, the new potential energy surface is not only recommended for dynamics studies of the F + H2 reaction in depth, but it can also be a building block for constructing the PESs of larger fluorine/hydrogen containing systems. In addition, the cross sections for the reaction F + H2 have been calculated as a function of collision energy from threshold value to around 10 kcal/mol. The comparison with other PESs is shown in Fig. 8 of Ref. [43]. In a word, the new PES is precise and more details about the PES could be found in Ref. [43].
The DCSs offer an excellent opportunity to study the most familiar vector correlation between the reagent and product relative velocity (k– k′ ). The DCSs for the reactions F + H2/HD/HT→ FH + H/D/T (v = 0, j = 0) at the selected collision energy of 2.74 kcal/mol are shown in Fig. 2. Clearly, the HF molecules are mainly scattered in the backward hemisphere, which is in a good agreement with the experimental angular distribution and QM calculations.[44– 48] In addition, we find that along with the quality increment of the target molecules (H2→ HD→ HT), the phenomenon of backward scattering is more pronounced. Therefore, it can be concluded that the isotope effect becomes more obvious with the reagent molecule H2 turning into HD and HT.
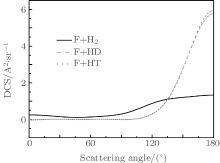 | Fig. 2. The DCS for the reactions F + H2/HD/HT→ FH + H/D/T as a function of scattering angle θ at the collision energy of 2.74 kcal/mol. |
To obtain a clearer understanding of the reaction mechanism, the variation of internuclear distances of atoms of reactants have been presented as a function of propagation time based on selected angle θ = 155.3° at 2.74 kcal/mol (see Fig. 3). It shows that the F atom collides with the HH/HD/HT molecule and forms the FH that moves away immediately, which can be concluded that this process consists with the direct dynamics. Therefore, it is no surprise that backward scattering has been observed as can be seen in Fig. 2. In addition, for the F + HD reaction, it should be noted that the distance of produce H and D atom happens in oscillation, which may be explained that the produce H atom rotates around the F atom.
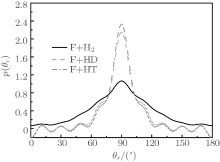
The relative alignment of the k and j′ vectors and the relative orientation of the k, k′ , and j′ vectors could be described by P(θ r) and P(ϕ r) distributions, respectively, which can provide an overall representation of the polarization of rotational angular momentum vector in the scattering plane. The distribution of P(θ r) for the product HF molecule in the reactions F + H2/HD/HT→ FH + H/D/T is shown in Fig. 4. The P(θ r) distribution is symmetric with respect to 90° and corresponding maximum is at 90° . The results demonstrate that j′ is distributed with cylindrical symmetry in the product scattering frame and the direction of j′ is perpendicular to the k direction preferentially. Furthermore, the peaks of P(θ r) display that the degree of the rotational alignment of the product increases with the qualitative increment of molecule targets. The peak of the P(θ r) distribution for F + H2 → FH + H reaction is the weakest, and for F + HT→ FH + T reaction is the strongest. It can be concluded that the enhancement of the quality of the reactants will strengthen the degree of the rotational alignment.
The P(ϕ r) distribution describing the k– k′ – j′ correlation is revealed in Fig. 5. It can be clearly seen that the P(ϕ r) distribution tends to be asymmetric with respect to the k– k′ scattering plane (or about ϕ r = 180° ). The peaks in the P(ϕ r) distribution at ϕ r angles close to 270° implies a preference for left-handed product rotations, while the peaks at ϕ r angles close to 90° indicates a preference for right-banded product rotation. The peaks at ϕ r = 90° and ϕ r = 270° indicate that the rotational angular momentum vector of the products is not only aligned, i.e., the distributions have peaks at ϕ r = 90° and 270° , but also oriented along the y axis of the CM frame. In addition, because the peak at ϕ r = 90° is much lower than that at 270° , j′ is more inclined to be oriented along the negative direction of the y axis. That is to say, the strong polarization of product rotational angular momentum could be demonstrated. If we use the term “ in-plane” to refer to this preference of the product HF to rotate in planes parallel to the scattering plane, and the term “ out-of-plane” to refer to the preference perpendicular to the scattering plane, we can conclude that the reactions are mainly governed by the “ out-of-plane” mechanism.
In order to show the angular momentum polarization clearly, the distribution of P(θ r, ϕ r) has been calculated (see Fig. 6), which represents the scattering angle average of the full distribution P(ω t, ω r). The tomographical features of the distributions of the P(θ r, ϕ r) peaks are at (π /2, 3π /2), which are in good accordance with the distributions of P(θ r) and P(ϕ r). It further demonstrates that the product rotational angular momentum j′ is not only aligned, but also oriented along the direction perpendicular to the scattering plane. In addition, the plot of the distribution of P(θ r, ϕ r) indicates that the reactions are mainly dominated by “ out-of-plane” mechanisms.
In summary, in the present work, the QCT method has been adopted to investigate the stereodynamics of the reactions F + H2/HD/HT→ FH + H/D/T on the12A’ FH2 ground state PES. 100000 trajectories have been run at the collision energy of 2.74 kcal/mol. The results of the calculated DCSs demonstrate that the k– k′ angular distribution is dominated by backward scattering, and the degree of the scattering will increase with the increase of reactant molecular quality. The direct dynamics processes can be concluded. The three angle distributions P(θ r), P(ϕ r), and P(θ r, ϕ r) have also been calculated. It shows that the product rotational angular momentum j′ is not only aligned, but also oriented along the direction perpendicular to the scattering plane. The calculated distribution of P(θ r, ϕ r) indicates that the FH product is preferentially polarized perpendicular to the scattering plane and the reactions are mainly dominated by “ out-of-plane” mechanisms.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|
| 43 |
|
| 44 |
|
| 45 |
|
| 46 |
|
| 47 |
|
| 48 |
|