†Corresponding author. E-mail: h-sun@cauc.edu.cn
*Project supported by the Doctoral Fund of Henan Polytechnic University, China (Grant Nos. B2013-013 and B2013-044) and the Research Projects of Science and Technology of the Education Department of Henan Province, China (Grant Nos. 14B430026 and 12A430010).
This paper reports the crystal growth of diamond from the FeNi–Carbon system with additive phosphorus at high pressures and high temperatures of 5.4–5.8 GPa and 1280–1360 °C. Attributed to the presence of additive phosphorus, the pressure and temperature condition, morphology, and color of diamond crystals change obviously. The pressure and temperature condition of diamond growth increases evidently with the increase of additive phosphorus content and results in the moving up of the V-shape region. The surfaces of the diamonds also become coarse as the additive phosphorus added in the growth system. Raman spectra indicate that diamonds grown from the FeNi-phosphorus-carbon system have more crystal defects and impurities. This work provides a new way to enrich the doping of diamond and improve the experimental exploration for future material applications.
It is well known that diamonds have many outstanding physical and chemical properties, such as extreme hardness, optical transmission over a wide range, high thermal conductivity, good insulator, so on.[1– 4] Some of these properties may be influenced by the impurities incorporated in the diamond lattice. Diamond crystals with some additive elements, like boron, nitrogen, and sulfur, are usually synthesized by the high pressure high temperature (HPHT) and chemical vapor deposition (CVD) growth methods.[5– 9] Diamonds with additive phosphorus exhibiting n-type semiconductor properties have been produced by CVD methods.[10]
Diamond can be synthesized from the phosphorus-carbon system at 6.3– 7.5 GPa and 1400– 1850 ° C, [11] and it is confirmed that these diamonds contained phosphorus as the impurity in the crystal lattice. However, these obtained diamond crystals using phosphorus as catalyst were too irregular and small to measure their physical and chemical properties. Consequently, it is very important to grow diamond crystals, > 2 mm in size, which is large enough to measure these properties. On the other hand, experimental studies and data are quite limited on the crystal growth of diamond crystals with phosphorus as an additive under high pressure and high temperature conditions.
For this purpose, growth experiments of diamond crystals were carried out at high pressure and high temperature conditions using additive phosphorus in the system of FeNi-carbon (FeNi-C). The diamond crystals were characterized by optical microscopes (OM), scanning electron microscope (SEM), and Raman spectroscopy. This work will provide a new way to enrich the doping of diamond and improve the experimental exploration for material applications in future.
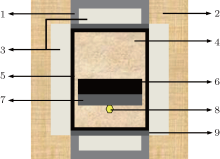
Growths of diamond crystals from the FeNi-phosphorus-carbon (FeNi-P-C) system were performed by the temperature gradient method using a China type cubic high pressure apparatus (CHPA) (SPD-6× 1200). The sample assembly for crystal growth of diamond is shown in Fig. 1. The FeNi alloy (Fe:Ni = 64:36, weight ratio, and 99.9% , purity) was used as the solvent catalyst. High purity graphite powder (99.9% , purity) was selected as a carbon source. Red phosphorus (purity 99.9% ) was used as an additive. High quality diamond crystals were used as seed crystals, with one of {100} crystal faces of about 0.7 mm× 0.7 mm in size as the initial growth crystal face. The growth time of diamond crystal was about 20.0– 21.0 h. In these experiments the pressure was estimated by the load of the oil press, which was calibrated by a curve that was established on the pressure-induced phase transitions of elements of Bi, Tl, and Ba, and temperature was determined by a Pt6% Rh– Pt30% Rh thermocouple, respectively.
Samples taken from the high pressure apparatus were placed in a boiling solution of nitric acid and sulfuric acid to remove the remaining graphite and metal catalyst. OM and SEM were used to observe the color, morphology, inclusions and surface characteristics of the diamond crystals. Raman spectroscopy was used to characterise the quality of the diamonds.
To investigate the influences of additive phosphorus on pressure and temperature conditions of diamond growth, the experiments were performed under the conditions of pressures and temperatures at 5.4– 5.8 GPa and 1280– 1360 ° C. The relationship between the growth conditions (pressure and temperature) of diamond crystals and the amount of additive phosphorus in the FeNi-P-C system is shown in Table 1. It can be seen that the pressure and temperature ranges of diamond growth change narrow evidently (N1, N2, and N3) with the increase of additive phosphorus. In the absence of phosphorus in the growth system at 5.4 GPa (N1), the temperature range is about 1280– 1320 ° C, and the morphologies of diamond crystals show that they are mainly composed of (100) crystal faces, (100)+ (111) crystal faces, and (111) crystal faces, respectively. However, in the presence of phosphorus in the growth system at 5.4 GPa (N2), the morphologies of diamond crystals show (100)+ (111) crystal faces, and (111) crystal faces. It is difficult to grow a diamond crystal that is mainly composed of (100) crystal faces. If we expand the range of temperature of crystal growth, it should raise the growth pressure, such as 1320– 1360 ° C at 5.6 GPa (N3), and 1350– 1360 ° C at 5.8 GPa (N4).
| Table 1. Experimental results of diamond growth from the FeNi-P-C system (“ – ” : no single crystal). |
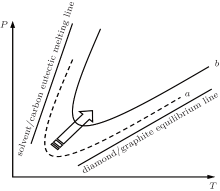
Generally, in the Pressure– Temperature P– T phase diagram of carbon, the district of diamond growth is a V-shape region bounded by solvent– carbon eutectic melting line and diamond– graphite equilibrium line in the metal solvent– carbon system, which is shown in Fig. 2. The solvent– carbon eutectic melting lines are different for diverse solvent– carbon system.[2] In our experiments, the properties of FeNi alloy solvent changed with the addition of additive phosphorus into the growth system of diamond, which results in the moving up of the V-shape regions (curve a moves towards curve b in Fig. 2). The most probable reason is that the FeNi alloy solvent and additive phosphorus may interact with each other under high pressure and high temperature conditions, and the characteristics of FeNi alloy solvent have been changed. In general, this change may increase the pressure and temperature conditions of crystal growth, and increase the energy required to grow the diamond.[8, 12, 13] Our research results are consistent with this.
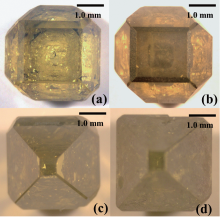
According to the above results, the pressure and temperature conditions for diamond growth obviously changed with the increase of additive phosphorus content. Figure 3 shows photographs of the diamond crystals from the FeNi-C system with different contents of additive phosphorus. Figure 3(a) shows the diamond crystal obtained in the absence of additive phosphorus. The crystal is yellow in color and has nearly no inclusions. As shown in Fig. 3(b), the diamond obtained in the presence of 0.5 wt% additive phosphorus, the crystal is dark in color and visible inclusions trapped in diamond crystals can be found easily. When additive phosphorus is up to 1.0 wt% , the obtained diamond is a deep dark color and has a bad transparence, which is shown in Fig. 3(c). At the same time, the content of inclusions increases with the increase of additive phosphorus. It can be seen in Fig. 3(d) that when the content of additive phosphorus is up to 1.5 wt% , the obtained diamond is of poor quality with more inclusions.
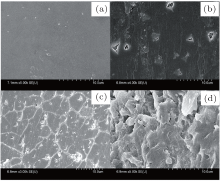
The surface characteristics of the diamond crystals from the FeNi-P-C system were analyzed by SEM. The SEM photographs of diamond crystals with different contents of additive phosphorus are shown in Fig. 4. It can be obviously seen that the integrality of diamond surfaces has been destroyed due to the introduction of additive phosphorus in the FeNi-C system. The surface of diamond crystal is smooth with a few pits (Fig. 4(a)), in which the growth system is in the absence of additive phosphorus. As shown in Fig. 4(b), there are more pits. A netlike growth texture appears as the additive phosphorus grows up to 1.0 wt% , as can be seen in Fig. 4(c). When additive phosphorus increases up to 1.5 wt% , the surface of diamond crystal becomes rougher, which is shown in Fig. 4(d). That is to say, the introduction of additive phosphorus reduced the quality of the diamond crystals. We believe that these results can be explained as follows. Some phosphorus dissolved in the FeNi solvent catalyst and made this alloy composition change. FeNi solvent catalyst with additive phosphorus was easily embedded in diamond crystals and formed the inclusions. The inclusions on the surface of diamond crystal were dissolved into the solution of nitric acid and sulfuric acid, and so pits remained on the diamond surfaces. At the same time, the appearance of pits on diamond surface would be more serious with the increase of phosphorus content in the growth system.
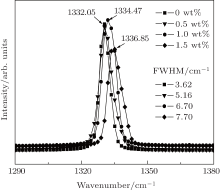
Raman spectroscopy was used to characterize the crystal defects or crystalline quality of diamond crystals. Figure 5 shows the Raman spectra of recorded diamond crystals from the FeNi-C system with different contents of additive phosphorus. It can be seen that the Raman spectra exhibits a series of system changes with the increase of phosphorus content in the growth system. Firstly, the positions of the maximum of Raman peaks tend to shift toward higher frequencies. The position of the maximum of the Raman peak without additive phosphorus is similar to that with 0.5 wt% additive phosphorus, which may be caused by the measurement error. However, the position of the maximum of the Raman peak with 1.0 wt% additive phosphorus is higher than that with 0.5 wt% . Secondly, the full width at half-maximum (FWHM) is about 3.62 cm– 1 for diamond grown in the system without additive phosphorus, 5.16 cm– 1 for diamond grown in the system with 0.5 wt% additive phosphorus, 6.7 cm– 1 for diamond grown in the system with 1.0 wt% additive phosphorus, and 7.7 cm– 1 for diamond grown in the system with 1.5 wt% additive phosphorus. The FWHMs of the diamond crystals become broader with the increase of the content of additive phosphorus. It is supposed that the observed features of the Raman spectra of diamond crystals from the FeNi-P-C system are related to impurities, residual stress, or defects, etc. Therefore, this illustrates that the diamond crystals grown from the FeNi-P-C system have more crystal defects and the quality is poorer than those from ordinary FeNi-C system.
Diamond crystals have been synthesized in the FeNi-carbon system with additive phosphorus at high pressure and high temperature conditions. The pressure and temperature conditions of diamond synthesis increase with the increase of phosphorus content and this results in the moving up of the V-shape region. Pits or netlike growth texture appear on the surfaces of the diamond and inclusions are trapped easily in diamond crystals. Raman spectra indicate that the diamond crystals grown from the FeNi-C system with additive phosphorus have more crystal defects and the quality is poorer than those from an ordinary FeNi-C system. Next, we need to improve the synthetic process, enhance the quality of diamond crystals, and then characterize the physical and chemical properties of the synthesized diamond with additive phosphorus.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|