†Corresponding author. E-mail: hqxie@eed.sspu.cn
*Project supported by the National Natural Science Foundation of China (Grant No. 51206103), the Innovation Program of Shanghai Municipal Education Commission, China (Grant No. 13YZ128), the Opening Project of CAS Key Laboratory of Materials for Energy Conversion, China, and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher learning, China.
In order to study the thermoelectric properties of TiO2-based hybrid materials, TiO2/polyparaphenylene (PPP) nanocomposites are fabricated by spark plasma sintering (SPS). The results show that the electrical conductivity follow percolation theory is enhanced due to the electron transfer highway provided by the conducting PPP phase. Furthermore, the thermal conductivity is reduced due to the drastic difference of vibrational spectra between organic and inorganic components. As a result, the greatest ZT = 0.24 is obtained for TiO2/0.75 wt% PPP sample, which is 15-fold higher than pure TiO2 (ZT = 0.016).
Thermoelectric materials have attracted renewed interest for their application in clean energy-conversion systems. Especially, vast amount of waste heat are used for thermoelectric power generation. The thermoelectric conversion efficiency is determined by the dimensionless figure of merit (ZT), defined as ZT = S2σ T/κ , where S is the Seebeck coefficient, σ is the electrical conductivity, T is the temperature, and κ is the thermal conductivity, respectively. Transition metal oxide ceramics, such as ZnO, [1] NaCo2O4, [2] NiO, [3] and TiO2, [4] have attracted more and more attention owing to their good thermoelectric prospects and excellent stability under high temperature. TiO2 might be considered as the prime candidate because of its large Seebeck coefficient and excellent thermal insulation property. Thurber et al.[5] reported that large negative Seebeck coefficients from − 1000 to − 200000 μ V/K have been observed at low temperature in rutile and Nb-doped rutile TiO2. Form the expression of ZT, we can know that a high-ZT thermoelectric material need to simultaneously achieve high electrical conductivity (σ ), large Seebeck coefficient (S), and low thermal conductivity (κ ) in the same solid. However, most oxides, including TiO2, are known to be poor electrical conductivity. Therefore, it has been an important issue of how to improve the electrical conductivity and maintain the Seebeck coefficient and thermal conductivity. In the past few decades, nanocomposites consisting of metal particles embedded in an insulating matrix have drawn great interest. For example, Xu et al.[6] prepared Al-doped TiO2 nanostructured materials to increase the electrical conductivity and achieved an enhanced ZT of 0.091; Liu et al.[7] reported that Ag-codoped TiO2 nanostructured materials exhibit an enhanced ZT of ∼ 0.082 at 1073 K. Unfortunately, the ZT value of TiO2 is still lower than the traditional thermoelectrics, such as Bi2Te3, [8, 9] PbTe, [10, 11] and GeSi[12, 13] alloys.
Most recently, inorganic-polymer hybrid materials have been paid much attention due to their unique properties. Poly(3, 4-ethylenedioxythiophene)-tellurium[14] and poly(3-hexylthiophene)-Bi2Te3[15] nanocomposites were prepared and achieved an enhanced ZT value. Inspired by those precursory result, we consider the fact that the electrical conductivity of TiO2 is lower, and incorporate a high electrical conductivity polymer into TiO2 matrix. On the one hand, it can increase the electrical conductivity; on the other hand, the drastic mismatch in characteristic vibrational spectra between organic and inorganic can lower the vibrational heat conductance.[15] In this study, a series of TiO2 nanoparticles were fabricated by sol-gel method. Then the as-prepared nanopartcles were mixed with polyparaphenylene (PPP) nanoparticles uniformly by ball milling. Finally, the mixed nanocomposite powder was compressed into bulk materials through spark plasma sintering (SPS). The effects of PPP in TiO2 are further investigated.
Titanium dioxide nanoparticles were prepared by a sol-gel method. In the preparation, 10 ml titanium alkoxide, as the raw material, is mixed with 40 ml 2-propanol in a dry atmosphere. This mixture was then added drop wise into another mixture consisting of 10 ml water and 10 ml 2-propanol. The acidity alkalinity of the gel was adjusted to pH 9. A yellowish transparent gel was formed after 1 h stirring. The obtained gel was then dried at 105 ° C for several hours until it turned into a yellow block crystal. Calcinations of the synthesized materials were carried out in air at 500 ° C for six hours in a Fischer Scientific furnace. The most widely employed PPP synthesis method is developed by Kovacic, in which the polymerization of benzene takes place in the presence of a catalyst, aluminum chloride (AlCl3), and an oxidant, cupric chloride (CuCl2). The obtained powder exhibits a brown color.
To study the thermoelectric properties, the TiO2 and PPP powders were weighted according to the ratio TiO2/x wt% PPP (x = 0, 0.5, 0.75, and 1), and loaded into the stainless steel ball-milling jar. The jar was subjected to ball milling for 20 h at 400 rpm. The ball-milled powders were consolidated by spark plasma sintering equipment (SPS-2040) at 1173 K under a pressure of 40 MPa in vacuum for 10 min.
Then, 2 × 2 × 10 mm3 bars and Φ 6 × 1.5 mm2 plates were cut to measure their thermoelectric properties. Both electrical conductivity and Seebeck coefficient were measured simultaneously using ZEM-3 system; the thermal conductivity was calculated from the value of the thermal diffusivity (λ ), density (ρ ), and specific heat (Cp) using the relationship κ = λ ρ Cp, in which λ was measured using a laser flash system (FL-4100), and Cp was measured on the Netzsch DSC 404C The phase and composition of the products were characterized by X-ray diffraction (XRD) using a Rigaku D/max 2500 diffractometer at a voltage of 40 kV and a current of 200 mA with Cu– Kα radiation (λ = 1.5406 Å ), employing a scanning rate of 0.02° /s in the 2θ ranging from 10° to 80° . Their microstructures were investigated by scanning electron microscope (SEM) and a transmission electron microscope (TEM).
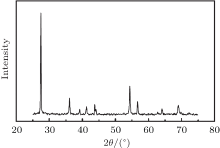
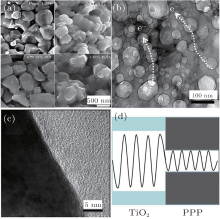
The X-ray diffraction patterns for TiO2/PPP nanocomposite recorded at room temperature are shown in Fig. 1. The diffraction peaks and relative intensities of all patterns match well with the rutile phase, no extra peaks of impurity phase were observed in the patterns. The PPP is an amorphous nature showing no obvious diffraction peaks. Figure 2(a) is the SEM image of TiO2/0.75 wt% PPP. It can be seen that there is irregular carpolite inside with dark contrast and outer with lighter contrast. The lighter-contrast area is PPP and the dark-contrast area is the polycrystalline of TiO2. This result can be deduced from our previous study.[16] Figure 2(b), which is representative TEM image, shows that the PPP is mainly located on the surface of TiO2 particles and the size is about 50– 100 nm. Besides, we can find some highways for transferring electrons labeled with white dotted arrow because the conducting polymer PPP has high ductility and toughness. Nevertheless, there is significant difference in characteristic vibrational states between semiconductor TiO2 and polymer PPP, as shown in Fig. 2(d), which is useful to reduce the thermal conductivity.
The temperature dependence of the electrical conductivity of the prepared composites is shown in Fig. 3(a). From the figure we can see an interesting phenomenon that hybrid conductivity increases slowly with the increase of PPP content when the PPP content is lower than 0.5 wt% and higher than 0.75 wt%. While the conductivity increases abruptly when the PPP content is between 0.5 and 0.75 wt%, exhibiting a typical semiconductor– conductor transition The electricity conductivity increase from 231 S· m− 1 for 0.5 wt% PPP sample to 2638 S· m− 1 for 0.75 wt% PPP sample at 300 K, corresponding to a 10-fold enhancement. It may be ascribed to the percolation theory.[17, 18] When the content of PPP reaches a certain value, it will help to build interconnected network as effective conducting paths which dominate the electrical conductivity of the composite rather than TiO2 matrix.[17] Besides, since the PPP– TiO2 junction leads to charge redistribution, the resulted deflexed energy band of TiO2 should facilitate the electron transfer across the interface, and thus accelerates the mobility of charge carriers. The behavior of Seebeck coefficient (S) as a function of temperature is displayed in Fig. 3(b), and the negative value of S indicates that all the samples are n-type semiconductors. The absolute value of the Seebeck coefficient shows an opposite trend to electrical conductivity, and drastically decreases with the increase of PPP content. Similar to the electrical conductivity, there is a big difference. The absolute value of Seebeck coefficient decreases with temperature when the PPP content is lower than 0.5 wt%, while the absolute value increases when the PPP content is lower than 0.75 wt%. According to the percolation theory, the reason for the abrupt increase in conductivity when the PPP content is between 0.5 and 0.75 wt% is mainly by the sudden improvement of charge carrier’ s mobility. The variety of charge carrier’ s mobility causes the change of the absolute value of Seebeck coefficient trend.
 | Fig. 3. Temperature dependence of the electrical conductivity (a), Seebeck coefficient (b), thermal conductivity (c), and ZT value (d) for the TiO2-based hybrid materials. |
The temperature dependence of the thermal conductivity for all the nanocomposites is shown in Fig. 3(c). Increased nanoparticle concentration of PPP results in an almost uniform decrease in thermal conductivity over the entire temperature range and the decrease extent is enhanced with the increase of PPP content. Compared with the pure TiO2 sample, the decrease extent is 15%, 20%, and 35%, respectively. The depression in thermal conductivity should be mainly attributed to three factors. Firstly, the drastic mismatch in characteristic vibrational spectra (Fig. 2(c)) between organic PPP and inorganic TiO2 can lower the vibrational heat conductance.[19] Secondly, the diameter of PPP particles is nanoscale, which can effectively scatter phonon.[20, 21] Finally, phonon scattering is due to the interface imperfections and boundaries.[22– 24] The temperature dependence of the thermoelectric figure of merit (ZT) is shown in Fig. 3(d). Compared to the pure TiO2 matrix, a dramatic improvement in ZT is observed for 0.75 wt% PPP sample due to the integrated effect of the electrical and thermal properties. As a result, the maximum ZT reaches 0.24, which is about 15 times higher than that of pure TiO2. This value is higher than that (ZT1073 K = 0.082) reported by Liu et al. concerning Ag0.05– Ti0.94Nb0.06O2.
In summary, a series of TiO2/PPP hybrid materials have been fabricated through spark plasma spark. Both the microstructure and thermoelectric properties of TiO2/PPP hybrid materials were investigated. The conductivity of the hybrid materials follows the percolation theory and is greatly enhanced due to the carrier transfer highway provided by the conducting PPP phase. At the same time, the thermal conductivity is significantly reduced due to the drastic mismatch in characteristic vibrational spectra between organic PPP and inorganic TiO2. An enhanced ZT value of 0.24 is obtained for TiO2/0.75 wt% PPP sample. This ZT value is one of the best ones reported for n-type TiO2-based thermoelectric materials.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|