†Corresponding author. E-mail: liurd104@163.com
‡Corresponding author. E-mail: fwwang@aphy.iphy.ac.cn
*Project supported by the National Basic Research Program of China (Grant No. 2010CB833102) and the National Natural Science Foundation of China (Grant Nos. 10974244, 11274369, and 11104337).
In this paper, multiferroics and magnetocapacitive effect of triangular-lattice antiferromagnet AgAl0.02Cr0.98S2 are investigated by magnetic, ferroelectric, pyroelectric current and dielectric measurement. We find that it is a multiferroic material and the magnetocapacitive effect reaches a factor of up to 90 in an external field of 7 T. The results imply the further possibility of synthesizing the magnetocapacitive materials by modifying the frustrated spin structure in terms of a few B-site doping nonmagnetic ions.
Multiferroic materials, in which two or three ferroic order parameters [ferroelectricity, (anti)ferromagnetism, and ferroelasticity] coexist, have been intensively investigated in recent years.[1– 5] Among them, the most attractive candidates for applications are those in which there exists a strong coupling between these parameters, especially magnetism and ferroelectricity. From a practical viewpoint, enhancing magnetoelectric coupling can realize mutual manipulation of the ferroic ordering parameters and multifunction, so exploring the correlation between magnetic and dielectric properties is very prerequisite for novel device applications. Recently, the multiferroic mechanism relating the magnetism to electrical polarization has been given for noncollinear magnets as Pi j = A0ei j × (Si × Sj). Here, ei j is the unit vector connecting neighboring spins, Si and Sj, and A0 a coupling constant related to the spin-orbit and spin-exchange interactions.[6] This model successfully explains the ferroelectricity of noncollinear magnetic ordering of transition metal oxides, such as TbMnO3, [7] Ni3V2O8, [8] CoCr2O4, [9] and MnWO4.[10] To obtain such a new multiferroic material with enhanced magnetoelectric coupling, modification or partial substitution of magnetic ions with nonmagnetic ions may be crucial to the applications of the noncollinear magnetic systems.
The triangular-lattice antiferromagnet AgCrS2 is one of the interesting candidates for such multiferroic materials.[11, 12] It crystallizes in the delafossite structure (space group R3m) at room temperature, described as a stack of layers of edge-sharing distorted octahedra CrS6 linked to irregular tetrahedra AgS4 along the c axis (Fig. 1(a)).[11– 15] AgCrS2 undergoes the superionic conductor-insulator transition at about 673 K.[14, 16] It experiences the paramagnet-antiferromagnet transition at around 41 K.[11, 12] In the past several years, AgCrS2 has been an intensively interesting subject as a multiferroic material due to the discovery of spontaneous electric polarization in the corresponding AgCrO2 oxide.[16] In this paper, we report that only 2% dilution of magnetic Cr3+ ions with nonmagnetic Al3+ ions in multiferroic AgCrS2 induces the colossal magnetocapacitance.
The polycrystalline sample of AgAl0.02Cr0.98S2 was prepared by standard solid-state synthesis method. High-purity powders of silver sulfide (Ag2S), chromium (III) sulfide (Cr2S3), and aluminium sulfide (Al2S3) precursors were weighted according to the relevant stoichiometric ratio. The resulting powder was carefully ground, pelletized, and sealed into an evacuated quartz tube. The tube was heated at 1175 K for 50 h. At room temperature, the phase composition and structure of the obtained sample were checked by powder x-ray diffraction (XRD), using a RINT 2400 diffractometer from Rigaku Corporation with graphite monochromatized Cu Kα radiation.
The direct-current (DC) magnetic susceptibilities were measured under an applied field of 0.1 T in a temperature range of 3 K– 400 K by a SQUID magnetometer (Quantum Design, MPMSXL-7). For electric measurements, the sample was cut into a thin plate with about 4 mm × 5 mm × 0.4 mm in size, and the electrodes were formed on both sides of the plate by heat-treatment silver paste. The dielectric measurement was conducted at five different frequencies (0.5, 1, 5, 10, and 100 kHz) using an LCR meter (Hioki-3532-50). To deduce the electric polarization, we measured the pyroelectric current with a constant rate of temperature sweep (3 K/min) using an electrometer (Keithley 6517B) and then integrated it with respect to time. The electric hysteresis loops of the sample were measured by Radiant Technologies Premier II system. The controlling of the sample temperature and the external magnetic field was ensured by the SQUID magnetometer. The electric field was applied perpendicularly to the magnetic field in all electrical measurements.
Figure 1(b) shows the expected XRD patterns of AgCrS2 and the experimental XRD patterns of AgAl0.02Cr0.98S2 with some preferred orientations at room temperature, confirming that the sample exhibits single-phase characteristic in the detection limit of XRD. All the diffraction peaks can be indexed by the Joint Committee on Powder Diffraction Standards Card (the card: 73-2240) for rhombohedral acentric R3m AgCrS2, indicating that the crystal structure with 2% Al-doping remained. The ratio of the nonmagnetic Al3+ ion radius to the magnetic Cr3+ ion radius is 0.81 which is higher than 0.59 — the criterion of the ratio of the ionic radius of the solute to the ionic radius of the solvent of forming interstitial solid solution.[17] So, Al3+ ions must replace Cr3+ ions in the compound of AgCrS2.
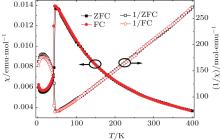
Figure 2 displays the temperature dependences of zero-field-cooled (ZFC) and field-cooled (FC) magnetic susceptibilities and the corresponding reciprocal magnetic susceptibilities of AgAl0.02Cr0.98S2 under an applied magnetic field of 0.1 T. Like the parent compound AgCrS2, [11, 12] the antiferromagnetic transition is observed at about 41.5 K, which is larger than that reported for the corresponding AgCrO2[18] oxide, TN = 21 K. The Né el temperature TN is not obviously changed by 2% substitution of Al3+ for Cr3+ in the compound AgCrS2. We fit the data from 150 K to 400 K by the Curie– Weiss law, and obtain that the effective Bohr magneton (μ eff) and the Weiss temperature (θ CW) are 3.70 μ B and – 59.12 K, respectively. The value of the effective Bohr magneton is almost consistent with the theoretically expected value of 3.87 μ B per Cr3+ like the parent compounds.[11– 13] The Weiss temperature is a negative value, indicating predominantly antiferromagnetic interaction. The frustration parameter value of the specimen (f = | θ CW| /TN) is 1.42, which is rather small compared with that of a strongly frustrated system with a value usually larger than 10.[19]
Figure 3(a) shows the electric hysteresis loops of AgAl0.02Cr0.98S2, measured at three temperatures (10 K, 25 K, and 45 K) with the E-sweeping frequency (f) of 1 Hz. Below 41.5 K the electric polarization cycles show up. Like the hysteresis loops of HgCr2S4, [20] they became more pronounced with reducing temperature. These indicate ferroelectric behaviors. The absolute value of the maximum polarization P is very close to that in CdCr2S4.[21] The ferroelectric hysteresis loops become constricted, indicating that the AgAl0.02Cr0.98S2 is a hard material. The experimental temperature is rather low and the shape of the electric polarization loop at 10 K is like a parallelogram, revealing that ferroelectric property can be regarded as being intrinsic. To further corroborate ferroelectric ordering in the doped AgCrS2, we measure the pyro-current to obtain information about the temperature dependence of electrical polarization (Fig.3(b)). Like the scenarios of AgCrS2 and AgCrO2, the polarization vanishes at ∼ TN, indicating that the doped AgCrS2 belongs in the spin-induced ferroelectrics.[12, 18] Recently, neutron scattering techniques evidenced a magnetoelastic-coupling-induced structural transition at TN in the parent compound AgCrS2.[11] These indicate that the ferroelectric loops and the electrical polarization originate from intrinsic ferroelectric property[22, 23] rather than the extrinsic Maxwell– Wagner effect.[24, 25]
 | Fig. 3. (a) Electric hysteresis loops for three temperatures performed at 1 Hz. (b) Electrical polarization versus temperature, measured after cooling in an electric field of 425 kV/m. |
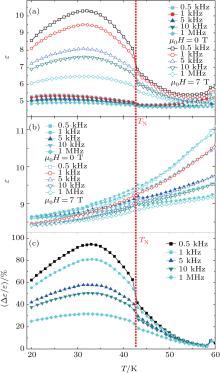
Figures 4(a) and 4(b) exhibit the temperature dependences of the dielectric constant without and with an applied magnetic field of 7 T at five frequencies (0.5 kHz, 1 kHz, 5 kHz, 10 kHz, and 100 kHz) for AgAl0.02Cr0.098S2 and the parent compound AgCrS2, respectively. Like HgCr2S4[20] and CdCr2S4, [21] the dielectric constants of the two compounds all depend greatly on not only temperature but also measured frequency. The dielectric constant of AgAl0.02Cr0.098S2 exhibits a slow rise below TN, and this is especially distinct under an external magnetic field of 7 T, which is different from the case of the parent compound. As illustrated in Fig. 4(b), the dielectric constant only has a small cusp near TN which is similar to the result reported in the literature, [12] and the external magnetic field affects the dielectric constant little. Compared with the dielectric constant of the parent compound, the values of dielectric constant decrease in the case without applied magnetic field. These changes should be caused by the magnetic defects — 2% substitution of nonmagnetic Al3+ ions for magnetic Cr3+ ions.
Figure 4(c) shows the temperature dependence of magnetocapacitance for the compound AgAl0.02Cr0.098S2. The magnetocapacitance, defined as relative change of dielectric constant, reaches a factor of up to 90. The magnetocapacitive effect of the compound depends on the measured frequency critically, which is similar to the scenarios of CdCr2S4 and HgCr2S4.[20, 21] Concerning the dielectric measurement, a prominent advantage of AgAl0.02Cr0.98S2 over the spinels CdCr2S4 and HgCr2S4 is due to the more insulating state of the former, [12] which avoids possible complications about the intrinsic origin of the colossal magnetocapacitance of the spinel.[26] To increase the influence of an external magnetic field on the arrangement of atomic magnetic moment, we destroy antiferromagnetic structure by substituting nonmagnetic ions Al3+ for magnetic ions Cr3+ partially, thereby the coupling of the magnetic property to dielectric property is enhanced. Of course, for a final reason further experiments are still necessary.
In the present work, the delafossite structure compound AgAl0.02Cr0.98S2 synthesized by the convenient solid reaction method, retain the multiferroics and magnetoelectric coupling effect. The magnetic defects — 2% nonmagnetic ions Al3+ substituting for the magnetic ions Cr3+ — induce the colossal magnetocapacitive effect, reaching a factor of up to 90, which may provide a most promising route to obtaining multifunctional material due to colossal magnetocapacitance.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|