†Corresponding author. E-mail: chunhuali@bjut.edu.cn
‡Corresponding author. E-mail: lijingyuan@ihep.ac.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 21273240, 11204267, and 11474013).
The intensive concern over the biosafety of nanomaterials demands the systematic study of the mechanisms underlying their biological effects. Many of the effects of nanomaterials can be attributed to their interactions with proteins and their impacts on protein function. On the other hand, nanomaterials show potential for a variety of biomedical applications, many of which also involve direct interactions with proteins. In this paper, we review some recent computational studies on this subject, especially those investigating the interactions of carbon and gold nanomaterials. Beside hydrophobic and π-stacking interactions, the mode of interaction of carbon nanomaterials can also be regulated by their functional groups. The coatings of gold nanomaterials similarly adjust their mode of interaction, in addition to coordination interactions with the sulfur groups of cysteine residues and the imidazole groups of histidine residues. Nanomaterials can interact with multiple proteins and their impacts on protein activity are attributed to a wide spectrum of mechanisms. These findings on the mechanisms of nanomaterial–protein interactions can further guide the design and development of nanomaterials to realize their application in disease diagnosis and treatment.
Because of their small size and/or nanostructure, nanomaterials often have a large specific surface area and produce quantum effects, [1, 2] which endow nanomaterials with a variety of unique physical and chemical properties, [3– 5] leading to their application in various areas including healthcare, electronics, cosmetics, and textiles. Consequently, the biological safety of nanomaterials has attracted much scrutiny.[6, 7] However, the potential threats of nanomaterials to human health, and the corresponding mechanisms, remain largely elusive. On the other hand, nanomaterials show significant potential for biomedical applications, e.g., drug delivery, diagnosis, bioimaging, and as therapeutic agents.[8– 14] Many of the biological effects of nanomaterials (both positive and negative) are related to their impacts on protein structure and function. Hence, the study of their interactions with proteins and their subsequent impact on protein function is crucial to gaining a better understanding of their biological effects. However, metabolism of nanomaterials is very complicated. For example, various proteins can adsorb on the surface of nanomaterials, forming a protein corona. The composition and content of the proteins within the corona are complicated and can continue to develop.[15] Hence, it is difficult to elucidate the mechanism underlying a given biological effect. In addition, relevant studies have often been hampered by the limitations of the spatial and temporal resolution of available experimental techniques.[16]
With the development of force fields and the improvement of sampling efficiency as well as computing power, computational methods have been widely used to study a variety of biological processes and drug designs. They have also been proven to be useful for research on the biological effects of nanomaterials. Using computational studies, the interactions between nanomaterials and proteins and their impacts on the structure and function of biological molecules can effectively be identified. Moreover, the findings of computational studies can guide the design and development of nanomaterials for biomedical applications, including disease diagnosis and treatment.
Carbon nanomaterials, including graphene, fullerene, and carbon nanotubes, are some of the most important inorganic nanomaterials. A variety of carbon nanomaterials exhibit diversified bioapplications, such as drug and gene delivery, contrast agents, therapeutic agents, and components of biosensors.[17– 21] However, inhalation of carbon nanomaterials may lead to stress, inflammation, lung insult, and a variety of cardiovascular effects.[22– 28] A growing number of computational studies have investigated the mechanisms underlying this spectrum of biological effects. Because their structure is well defined, carbon nanomaterials also serve as representative hydrophobic nanomaterials in pioneering studies of protein– nanomaterial interactions. To the best of our knowledge, one of the first simulations of protein– nanomaterial interactions involved the study of the binding of fullerene C60 to an antibody.[29] Ma and co-workers found that C60 interacts with the antibody, forming a complex with high affinity and specificity. The binding can be attributed to shape complementarity and extensive side-chain interactions, including hydrophobic and π -stacking interactions. Because this binding mode is similar to that of many other protein– ligand complexes, C60 may competitively interact with protein-binding sites and disturb protein– ligand recognition.
Fullerene consists of carbon cages with a diameter of approximately 1 nm. It has been widely exploited in the fields of bioimaging, drug delivery, and antitumor therapy, [30– 32] but the poor aqueous solubility of fullerene poses challenges for further applications. Various fullerene derivatives have been developed, for example, fullerene can be hydroxylated to fullerenol. Surface modification not only improves their solubility, but also endows them with additional features. Moreover, metal ions can be embedded in the carbon cage of fullerenol to form metallofullerenol. In general, metallofullerenol shows similar surface properties to fullerenol, but the embedded metal atom gives rise to several unique properties, [33– 36] as will be discussed below.
Experimental studies have shown that a typical endohedral metallofullerenol, Gd@C82(OH)22, can effectively inhibit tumor growth with low toxicity both in vivo and in vitro.[37, 38] After treatment with Gd@C82(OH)22, the thickness and flexibility of the fibrous layer surrounding the tumor significantly increases, leading to the formation of a fibrous cage that imprisons the tumor tissue and prevents metastasis. The increased thickness of the fibrous layer can largely be attributed to the suppression of the expression of matrix metalloproteinases (MMPs), as well as the reduction of their activities.[39, 40] To elucidate this important mechanism involved in the antitumor effect of metallofullerenol, Zhou and co-workers studied the interaction of Gd@C82(OH)22 with MMP-9 and its impact on protein function (Fig. 1(a)).[40] Metallofullerenol can firmly bind to proteins, but does not disturb their structure. Interestingly, instead of direct interactions with catalytic Zn2+ ions, Gd@C82(OH)22 was found to allosterically modulate the S1′ ligand-specificity loop, which might interfere with binding of the incoming substrate. In other words, metallofullerenol indirectly inhibits the activity of MMP-9. Moreover, the authors successfully characterized the complete binding process as having three stages, and identified the critical interactions of each stage.
 | Fig. 1. Interactions of Gd@C82(OH)22 with proteins. (a) (left) Representative binding mode (solid ball) as well as the alternative binding mode (gray ball) of Gd@C82(OH)22. (right) The binding dynamics can be characterized as having three phases. Adapted with permission from Ref. [40]. Copyright 2012 National Academy of Sciences, USA. (b) (left) Representative snapshot of a tropocollagen molecule bound by Gd@C82(OH)22. Snapshot of a tropocollagentetramer (middle) and a tetramer bound by Gd@C82(OH)22 (right). Reproduced from Ref. [46] with permission from The Royal Society of Chemistry. |
In addition to its impacts on fibrous layer thickness, Gd@C82(OH)22 was found to affect the structure and biophysical properties of collagen fibers, [39] the major component of the fibrous layer.[41– 45] The fibrous layer becomes softer after the treatment, and this is also an important antitumor mechanism of metallofullerenol. Under this consideration, we investigated the interaction of Gd@C82(OH)22 with molecular collagen (tropocollagen molecules), and its impact on protein structure and assembly (Fig. 1(b)).[46] Gd@C82(OH)22 can strongly bind to tropocollagen, largely due to hydrogen bond interactions with the protein. Adhered Gd@C82(OH)22 can enhance the stability of the native triple helical structure of tropocollagen and facilitate protein assembly. Interestingly, the interaction of nanoparticles with proteins has often been considered to disturb protein structure or to induce abnormal assembly; [47– 50] however, as indicated in our work, nanoparticles may also enhance the native structure and assembly of proteins. In the early stages of collagen fiber formation, Gd@C82(OH)22 can form hydrogen bonds with multiple tropocollagen molecules, acting as a “ fullerenol-mediated bridge” , and enhance interactions between collagen molecules during the course of fiber nucleation.[51– 56] However, during the growth of collagen fibers, metallofullerenol may interfere with interactions among proteins and affect the structure and stiffness of the collagen fibril layer.
Carbon nanotubes (CNT) are another widely used carbon nanomaterial, [57] and show similar challenges (e.g. poor solubility) and potential to their fullerene counterparts, such as drug design, drug delivery, tumor therapy, tissue engineering, DNA recognition, and biosensor design.[58– 62] In addition, CNTs are often used as representative hydrophobic nanoparticles to study the role played by hydrophobic interactions in protein– nanoparticle interactions, and their impact on protein structure and function.
Zhou and co-workers used WW domains (i.e., YAP65, YJQ8, and PIN1) as examples to study the interaction of CNT with proteins, and the subsequent impact on protein activity (Fig. 2(a)).[48] As signaling and regulatory proteins, WW domains can identify and bind to proline-rich motifs (PRMs).[63– 67] The authors found that CNT can plug into the hydrophobic core of WW domains because of their interactions with hydrophobic residues. More importantly, the binding of CNT blocks the PRM active site and thus hinders the interaction of PRM with the WW domain.
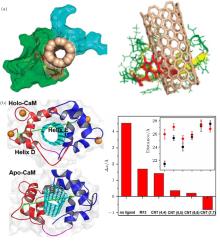 | Fig. 2. Impacts of carbon nanotube (CNT) on protein function. (a) (left) The binding of single CNT hinders the interaction between YAP65 (green) and its native ligand PRM (navy). (right) The interaction mode of CNT. The binding scaffold residues are highlighted as red sticks. Adapted with permission from Ref. [48]. Copyright 2010 American Chemical Society. (b) (left) Representative configuration of a CNT-CaM complex and a representation of the end-to-end distance of the inter-domain linker. (right) Difference in the end-to-end distance between two states. Inset: End-to-end distances of inter-domain linkers in different complexes. Reproduced from Ref. [68] with permission from The Royal Society of Chemistry. |
In addition to direct blockage of the active site, CNT can affect protein function via other mechanisms. Recently, [68] we investigated the binding of CNT to calmodulin (CaM) and its impact on the Ca2+ -dependent dynamic properties of CaM (Fig. 2(b)). In addition, we studied the size-dependence of the biological effect of CNT. CaM plays a crucial role in the calcium signal transduction pathway.[69] It can bind to a large variety of enzymes in a Ca2+ -dependent manner:[70, 71] Ca2+ facilitates ligand binding by enhancing hydrophobic interactions between ligand and protein, [72– 75] and ion removal triggers ligand dissociation. We found that CNT can recognize the hydrophobic binding pocket of CaM. Although small CNTs show behavior similar to that of the native substrate, the M13 peptide; in dissociation from Ca2+ -free CaM, wider CNTs continue binding to CaM in the absence of Ca2+ , indicating a potential failure of Ca2+ regulation and the inhibition of the calcium-dependent signal transduction pathway. This size-dependent impact on protein dynamic properties is largely due to the fact that wider CNTs show strong hydrophobic interactions with the protein and do not require the assistance of Ca2+ . Hence, the binding of wider CNT can dodge regulation by Ca2+ . The results of the simulation of CNT binding and the failure of Ca2+ regulation were confirmed by circular dichroism spectroscopy.
Graphene is a 2D plate-like carbon material with several extraordinary structural, mechanical, and electronic properties.[76] A growing number of investigations have explored the biomedical applications of graphene and its interactions with biological systems.[77] Zuo et al. used molecular dynamics simulation to investigate the adsorption of the protein villin headpiece (HP35) onto graphene.[78] The HP35 protein is composed of a three-helix bundle, and most of its native secondary and tertiary structures change after the adsorption. Its adsorption stability is largely attributed to π -stacking interactions between graphene and the aromatic residues of the protein. Moreover, because of its softness the shape of graphene can adapt to the distribution of aromatic residues and form strong π -stacking with proteins. The authors also compare the binding mode of graphene with those of CNT and C60, and found that the surface curvature of nanomaterials with identical chemical components can affect their mode of interaction with proteins. In addition to the study of protein adsorption, interactions between graphene and phospholipids, another important type of biological molecule, has attracted intensive attention. Zhou and co-workers found that graphene can extract phospholipids from the membrane, thereby destroying membrane structure.[79] In addition, Gao and co-workers systematically investigated the insertion and entry of graphene into membranes.[80]
Nanomaterials that contain noble metals possess a variety of unique physical properties, and they can serve as representative systems for study of the quantum confinement effect. Because of their stable chemical properties and high biocompatibility, gold nanomaterials have become one of the most popular noble metal nanomaterials. With the rapid progress in the synthesis and modification of gold nanomaterials, a wide spectrum of gold nanomaterials have been developed, including nanorods, nanoclusters, nanobelts, nanostars, and polyhedral nanoparticles. The distinct optical properties of gold nanomaterials engender potential applications in biomedical imaging, sensing, and photothermal therapy. Studies of the interactions of gold nanomaterials with biological molecules are currently in high demand.
Gold nanorods, AuNRs, are 10 nm– 20 nm in width and 10 nm– 100 nm in length. The optical adsorption of AuNR can effectively be regulated by changing their aspect ratio.[81] The optical properties of gold nanorods underlie their biomedical applications, and their potential impacts on living systems are attracting a great deal of attention.[82, 83] The protein corona that assembles on the surface of AuNR during metabolic processing modulates the biological response by mitigating its cytotoxicity, [84– 87] changing its biodistribution, [88– 90] and altering the inflammatory response.[91] Bovine serum albumin (BSA), the most abundant serum protein, is an important component of the corona. Study of its adsorption on AuNR is critical for understanding the formation of the protein corona. Previous experimental studies have shown that BSA can stably bind to the surface of AuNR and improve its biocompatibility, [92] whereas the detailed molecular mechanism and corresponding structure remain elusive. We combined experimental synchrotron radiation (SR)-based analytical techniques[93– 97] with molecular dynamics simulation to investigate the interaction of the BSA corona with AuNR (Fig. 3(a)). Binding of BSA is largely attributed to Au– S coordination between the gold atoms and sulfur atoms of the cysteine residues. There are 17 disulfide bonds between cysteine residues in BSA, most of which are exposed to the solvent. We found that 8 of the disulfide bonds are distributed on a plane, denoted as plane S. We further investigated the process of BSA adsorption by molecular dynamics simulation. The plane S serves as the binding interface, and at least 12 Au– S coordination bonds form during adsorption. These findings were confirmed by SR S K-edge x-ray absorption near-edge structures (XANES)[98] and SR-based microbeam x-ray fluorescence[99– 101] (XRF) results. In addition, our study indicates that such combined approaches are effective for study of the interfacial interactions of the protein corona with AuNR, which should improve our understanding of the protective effects of the corona.
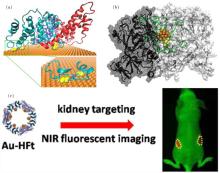 | Fig. 3. The interactions of gold nanoparticles with proteins. (a) The binding of BSA to the Au (111) surface of AuNRs. The three domains of BSA are shown in cyan, red, and blue; the disulfides are shown as yellow spheres. Adapted with permission from Ref. [92]. Copyright 2013 American Chemical Society. (b) The AuNC– TrxR1 complex. The gold atoms, peptide chain, and Cys/Sec residues of the active site are shown in orange, green, and yellow, respectively. Reproduced from Ref. [112] with permission from The Royal Society of Chemistry. (c) Designed near-infrared (NIR) fluorescent hybrid nanocomposite: multiple AuNCs within a cage of H-ferritin (HFt). This designed probe can realize kidney targeting and NIR-imaging of live animals. Adapted with permission from Ref. [110]. Copyright 2015 American Chemical Society. |
Gold nanoclusters, AuNCs, are composed of a few to roughly a hundred atoms with a diameter < 2 nm. The properties of AuNC are distinct to both isolated atoms and larger nanoparticles.[102] As a representative fluorescent nanomaterial, AuNC is attractive for biolabeling and bioimaging applications owing to its ultra-small size, nontoxicity, and highly fluorescent properties.[103– 105] To improve their suspension stability, AuNCs are often protected with various coatings such as alkanethiols, DNA, peptides, or even protein cages, [106– 109] resulting in bioinorganic hybrid nanomaterials. The coatings can also modulate the surface properties of AuNCs and improve their biocompatibility.
As indicated in previous work by our collaborators, a ferritin protein cage can be used to guide the formation of gold nanoclusters, producing a gold cluster-based hybrid nanocomposite.[110] The ferritin complex is composed of 24 monomers of two types, i.e., heavy chain and light chain (H- and L-ferritin, respectively). According to the results of our simulation, the nucleation sites of gold clusters are located in the His-rich surface region of H-ferritin, HFt. Interaction of gold atoms with the imidazole group of histidine facilitates the formation of gold clusters. Accordingly, our collaborators used protein nanocages solely composed of HFts to synthesize a hybrid nanocomposite containing 24 AuNCs. The number of nanoclusters within each nanocage was confirmed by cryo-electron microscopy imaging. Moreover, such Au-HFt nanocomposites can serve as near-infrared (NIR) probes with a high fluorescent yield, showing powerful tissue-penetrating abilities. Because ferritin shows a kidney-targeting ability, this designed NIR Au-HFt probe may be exploited for the diagnosis of kidney diseases in live animals (Fig. 3(c)).
More interestingly, AuNC can directly bind to target proteins and affect the protein activity, exhibiting potential for disease treatment. Our collaborators synthesized a peptide-coated Au25 cluster, [111] and found that the AuNC can specifically bind to thioredoxinreductase1 (TrxR1).[112] TrxR1 is important for regulation of cellular redox levels and is often overexpressed in cancer cells.[113, 114] The protein has been recognized as a potential target for anti-tumor therapeutic agents.[115, 116] Treatment with peptide-coated AuNC was found to effectively suppress protein activity, resulting in an increased concentration of reactive oxygen species and the subsequent apoptosis of tumor cells. The potential of peptide-coated AuNC in tumor therapy requires detailed study of the corresponding mechanism, especially the mode of interaction of AuNC with proteins. We used molecular docking methods[117] to search for binding siteson the surface of TrxR1 and successfully identified a putative binding region near the active site, Cys497-Sec498 (Sec, selenocysteine). Additional molecular dynamics simulations were then performed to assess the binding stability of AuNC (Fig. 3(b)). In general, AuNC can directly bind to the region near the active site, mainly due to electrostatic attraction between the positively charged coating peptides and the negatively charged surface residues near the active site. In addition, hydrogen bonds and hydrophobic interactions are involved in the binding of coated AuNC, which facilitates the subsequent coordination interaction of Au25 cluster with cysteine and selenocysteine.[118] Thus, AuNC can selectively recognize TrxR1 in vivo. The study of the molecular mechanisms underlying these biological effects may inspire the design of therapeutic gold nanoparticles against diseases involving TrxR1, such as cancer.
In this paper, we reviewed recent computational studies of how proteins interact with carbon and gold nanomaterials. The interactions between carbon nanomaterials and proteins can largely be attributed to hydrophobic and π -stacking interactions. Whereas binding of proteins to pristine gold nanomaterials mainly results from Au– S coordination as well as interactions with the imidazole group of histidine, gold nanomaterials are often modified by various coatings and coated gold nanomaterials also interact with nanomaterials via electrostatic, hydrophobic, and hydrogen bond interactions.
Interactions with nanomaterials often influence protein activity. For example, CNT can interact with the active site of the WW domain and hinder interactions with the native ligand. In addition, binding of CNT can interrupt the dynamic properties of CaM in a size-dependent manner. On the other hand, the influence of nanomaterials on protein structure and activity provides potential therapies for disease treatment. Metallofullerenol Gd@C82(OH)22 has been found to effectively inhibit tumor growth by increasing the thickness and flexibility of the fibrous layer of tumors to “ imprison” cancer cells. The anti-tumor effect of Gd@C82(OH)22 involves multiple target proteins. The expression and activity of matrix metalloproteinases are down-regulated by nanoparticles. In addition, Gd@C82(OH)22 can affect the structure and assembly of molecular collagen, reducing the stiffness of the collagen fibrous layer.
Because of the rapidly growing number of biomedical applications, the interaction of gold nanomaterials with proteins has also attracted widespread interest. The importance of Au– S coordination in such systems has been recognized, and the binding behavior of some proteins, which depends on the distribution of exposed cysteine residues, has been captured by experimental techniques such as S K-edge XANES. The binding of peptide-coated AuNC to the cysteine-containing active site of TrxR1 results in the inhibition of protein activity and the apoptosis of cancer cells. In addition, the imidazole group of histidine has also been found to be involved in protein interactions with gold nanoparticles. For example, the histidine-rich surface region of HFt can guide the nucleation and growth of AuNC.
The complicated nature of metabolic processing of nanomaterial poses challenges for studying their interactions with proteins in vivo. On the other hand, computational studies can be used effectively to investigate the mechanisms underlying protein– nanomaterial interactions and the subsequent impact on protein activity, and elucidate the mechanisms underlying their biological effects. As described above, nanomaterials often interact with multiple target proteins and the modulation of protein activity can be attributed to a wide spectrum of mechanisms. In addition, the results of simulations can further guide the design of nanomaterials to integrate functionality and/or enhance their desired properties.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|
| 43 |
|
| 44 |
|
| 45 |
|
| 46 |
|
| 47 |
|
| 48 |
|
| 49 |
|
| 50 |
|
| 51 |
|
| 52 |
|
| 53 |
|
| 54 |
|
| 55 |
|
| 56 |
|
| 57 |
|
| 58 |
|
| 59 |
|
| 60 |
|
| 61 |
|
| 62 |
|
| 63 |
|
| 64 |
|
| 65 |
|
| 66 |
|
| 67 |
|
| 68 |
|
| 69 |
|
| 70 |
|
| 71 |
|
| 72 |
|
| 73 |
|
| 74 |
|
| 75 |
|
| 76 |
|
| 77 |
|
| 78 |
|
| 79 |
|
| 80 |
|
| 81 |
|
| 82 |
|
| 83 |
|
| 84 |
|
| 85 |
|
| 86 |
|
| 87 |
|
| 88 |
|
| 89 |
|
| 90 |
|
| 91 |
|
| 92 |
|
| 93 |
|
| 94 |
|
| 95 |
|
| 96 |
|
| 97 |
|
| 98 |
|
| 99 |
|
| 100 |
|
| 101 |
|
| 102 |
|
| 103 |
|
| 104 |
|
| 105 |
|
| 106 |
|
| 107 |
|
| 108 |
|
| 109 |
|
| 110 |
|
| 111 |
|
| 112 |
|
| 113 |
|
| 114 |
|
| 115 |
|
| 116 |
|
| 117 |
|
| 118 |
|


