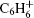†Corresponding author. E-mail: lvhang0811@sina.com
Corresponding author. E-mail: xuhf@jlu.edu.cn
*Project supported by the National Basic Research Program of China (Grant No. 2013CB922200) and the National Natural Science Foundation of China (GrantNo. 11274140).
Ionizations and fragmentations of benzene, methylbenzene, and chlorobenzene are studied in linearly polarized 50-fs, 800-nm and 400-nm strong laser fields using a time-of-flight mass spectrometer. It is shown that at low laser intensity, the parent ions are dominant for any one of the molecules in an 800-nm strong laser field, while extensive fragmentation is observed in a 400-nm laser field, which can be understood by the resonant photon absorption of molecular cations. The ratio of the yield of the parent ion to the yield of the total ion for each molecule is measured as a function of laser intensity in a range from 1.0 × 1013 W/cm2 to 4.0 × 1014 W/cm2, in either the 800-nm or 400-nm laser field. The results show that the fragmentation of the aromatic molecules increases significantly as the laser intensity is increased. Possible mechanisms for fragmentation in strong laser fields are discussed. Finally, the saturation intensity of ionization of the titled molecules is also determined.
The interaction of a strong laser field with molecules has aroused intense research interest during the past two decades.[1– 7] Owing to the complicated structure and additional nuclear degree of freedom, molecules exhibit much more complex processes than atoms when being exposed to a strong laser field. Efforts have been made to investigate the peculiar behaviors of different molecules to reveal the structure effect in the molecular strong-field physical processes. Particularly, ionization is the fundamental process of molecules in a strong laser field, which is of great importance to better understand the phenomena that result from the interaction of molecules with strong laser fields, such as Coulomb explosion, [8] alignment and orientation, [9– 12] coherent control, [13] and high-order harmonic generation.[14]
For polyatomic organic molecules such as benzene, a particular interest is the competition between ionization and fragmentation in a strong laser field. As is well known, in a nanosecond laser field, fragmentation is dominant with a small yield of parent ions in multiphoton ionization, which is called the ladder-switching fragmentation.[15] For a short-pulse laser field, the magnitude of the laser electric field is comparable to that of the Coulombic field of the electron within the target molecule, thus leading to different behaviors between ionization and fragmentation compared with those in a nanosecond laser field. Interestingly, for some polyatomic molecules, strong field ionization has been observed to produce a few fragmentations even when the laser peak intensity is over 1013 W/cm2. Various studies have shown that the photon absorption of the molecular cation plays an important role in producing the fragmentations.[16] If the laser wavelength is in resonance with the wavelength of the molecular cationic photon absorption, extensive fragmentation occurs in strong field ionization; otherwise, the parent ions are dominant. In addition, it has also been reported that other factors, such as molecular geometry, laser pulse width, laser peak intensity, and the condition of laser focusing, may also affect the strong field ionization and fragmentations of polyatomic organic molecules.[17]
Here, we experimentally study the strong-field ionizations and fragmentations of benzene and two substituted benzenes: methylbenzene and chlorobenzene. Although the ionizations of benzene and methylbenzene have been investigated mainly in 800-nm strong laser fields, [18– 20] a comparative study between benzene and substituted benzenes is still needed. Figure 1 shows the structures and the highest occupied molecular orbitals (HOMOs) of benzene, methylbenzene, and chlorobenzene. Although the ionization potentials (Ip) of the molecules are close, which are 9.24 eV, 9.08 eV, and 8.83 eV for benzene, chlorobenzene, and methylbenzene, respectively, their structures are quite different. Benzene is a symmetric top molecule which belongs to the D6h molecular point group. When a hydrogen atom in benzene is substituted by a methyl radical or a chlorine atom, the six-fold rotational symmetry is broken, and consequently the two-fold degenerate of the HOMO in benzene is lifted. Such a difference in structure may lead to peculiar behavior when the benzene and substitute benzenes are exposed to a strong laser field. In the study, by measuring the mass spectra of the titled molecules irradiated respectively by linearly polarized IR and UV laser fields in an intensity range from 1.0 × 1013 W/cm2 to 4.0 × 1014 W/cm2, we perform a comparative study of the strong field ionization and fragmentation of the three molecules. Our results may present more knowledge of the interaction of polyatomic organic molecules with strong laser fields.
The experimental setup used for femtosecond strong laser ionization and dissociation of the aromatic molecules has already been described in detail in our previous studies.[21– 23] Briefly, liquid benzene or a substitute benzene sample was vaporized at room temperature by being continuously and homogenously mixed with pure Ar gas. The gas mixture was introduced into the vacuum chamber through a leak valve to interact with the strong laser pulses. The stagnation pressure was kept at ∼ 1 atm, and the operating pressure in the chamber was about 3 × 10− 4 Pa. The femtosecond laser system was a Ti:Sapphire chirped-pulse amplified laser with a central wavelength of 800 nm, pulse duration of 50 fs, repetition rate of 1 kHz, and maximum pulse energy of 4 mJ. The fundamental 800-nm or its second harmonic 400-nm pulse was focused into the chamber to ionize the aromatic molecules. A half-wave plate and a Glan prism were inserted into the laser beam to vary the laser intensity continuously. The peak intensity of the focused laser pulse was calibrated by comparing the measured saturation intensity of Xe with that calculated by the Ammosov– Delome– Krainov (ADK) model. A linear time-of-flight (TOF) mass spectrometer operated under the Wiley– McLaren condition was used to detect the produced cations from strong-field ionization and dissociation. All the cations were extracted, accelerated, and finally detected by a dual microchannel plate (MCP) detector at the end of the flight about 55 cm. A slit with 0.5 mm width was mounted in front of the flight tube to ensure that only those ions produced in the central portion of the focused volume were detected. Mass-resolved ion signals were recorded using a digital oscilloscope (Tektronix TDS 3054B) and sent to a PC for analysis. All experimental data were normally averaged over 103 laser shots.
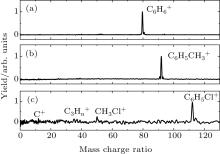
In this study, we perform a comparative study of the strong field ionizations and fragmentations of the titled aromatic molecules, irradiated by linearly polarized near-IR (800-nm) or UV (400-nm) laser fields. Figure 2 shows the measured mass spectra of benzene (Fig. 2(a)), methylbenzene (Fig. 2(b)), and chlorobenzene (Fig. 2(c)) recorded in a strong 800-nm laser field. The laser intensity is kept at 2.0 × 1013 W/cm2, corresponding to the Keldysh parameter γ value (γ = (Ip/2Up)1/2, where Up is the ponderomotive energy) of approximately 2.0 for all the three molecules. As shown in the figure, for benzene and methylbenzene, only the peaks of the singly charged parent ions, 


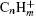
The results in Figs. 2 and 3 clearly show the different behaviors in the fragmentations of the molecules in a strong 800-nm laser field from those in the 400-nm laser fields. For some polyatomic molecules, it is indicated that the parent ions are dominant in the mass spectra if their cations have no absorption at the laser wavelength; on the contrary, if there is resonant absorption of the cations at the laser wavelength, the fragmentations make significant contributions to the strong field ionization with producing a small parent ion yield.[16, 24, 25] We calculate the absorption spectra of 



We further measure the values of ratio P+ /T+ for benzene and two substituted benzenes at laser intensities ranging from 1.0 × 1013 W/cm2 to 4.0 × 1014 W/cm2. The results are shown in Fig. 5, at 800-nm and 400-nm laser fields. The pattern of fragmentation in the mass spectra of each molecule remains unchanged in the whole laser intensity range, while the peak intensity of the fragment ions increases as the laser intensity is increased. For benzene and methylbenzene, the values of the P+ /T+ ratio at 800 nm are much larger than that at 400 nm in the whole intensity range; while for chlorobenzene, the values of the P+ /T+ ratio in the IR and UV laser fields become comparative at high laser intensity. For either of the molecules, the P+ /T+ ratio decreases as the laser intensity is increased, indicating the increased fragmentation in the process of strong field ionization. At 800 nm, the P+ /T+ ratio is 100% at low intensities, and the ratio values drop to about 33% and 15% at high intensity of 4.0 × 1014 W/cm2 for benzene and methylbenzene, respectively. The fragmentations of benzene and methylbenzene at high laser intensities are similar to those presented in Fig. 3, which are mainly composed of 
The increased fragmentations at high laser intensity could be attributed to two possibilities. Firstly, at high laser intensity, the electronic states of the molecules are so strongly disturbed by the laser field that the above-mentioned resonant photon-absorption effect of cationic eigenstates may become invalid. Thus further investigation of this issue should consider the laser-dressed electronic states of the cations. Secondly, in the tunneling ionization regime, the liberated electron via tunneling could be recollided with the ion core under the combined Coulomb potential and the oscillating laser electric fields. Such a recollision process, also known as the “ three-step rescattering” model, [28] can cause molecules to dissociate since the returned electron may carry large kinetic energy obtained from the strong laser fields. It is interesting to see from Figs. 5(a) and 5(b) that the P+ /T+ ratio between benzene and methylbenzene in an 800-nm laser field does not change until the laser intensity is increased to a threshold. The maximal kinetic energy Emax of returned electron (3.17Up) at the threshold laser intensity is about 4.4 eV for benzene and 3.8 eV for methylbenzene, which are very close to the dissociation energies of 
The measurement of the ionization saturation intensity proves to be useful for comparing the responses of different molecules to the strong laser field. In the present study, we use the approach reported by Hankin et al., which can determine well-defined saturation intensity (Isat) for polyatomic organic molecules.[28] In the approach the curve of the semi-logarithmic sum of all the fragment ion yields containing carbon atoms (Σ Cn) versus laser intensity is plotted. The Isat value can be derived by extrapolating the linear portion of the curve to the laser intensity axis. The results of benzene, methylbenzene, and chlorobenzene for the 800-nm laser field are presented in Fig. 6 and those for the 400-nm laser field in Fig. 7. The determined Isat values of the molecules are listed in Table 1. For comparison, in the table we also present the Isat value calculated by the ADK model (IADK) for a virtual atom with the molecule’ s Ip, and the ratio of Isat/IADK both for the 800-nm and 400-nm laser fields.
 | Fig. 6. Intensity dependences of all the fragment ion yields containing carbon atoms (Σ Cn) in an 800-nm femtosecond laser field for (a) C6H6, (b) C6H5CH3, and (c) C6H5Cl. |
 | Fig. 7. Intensity dependences of all the fragment ion yields containing carbon atoms (Σ Cn) in a 400-nm femtosecond laser field for (a) C6H6, (b) C6H5CH3, and (c) C6H5Cl. |
| Table 1. Saturation intensities of C6H6, C6H5CH3, and C6H5Cl, determined by experiment and ADK calculation and the ISAT/IADK values for 800-nm and 400-nm laser fields. |
As is well known, the strong field ionization probability of atoms can be well described by the ADK model.[30] Because of the similar Ip values, the IADK values of the virtual atoms are almost identical in both 800-nm and 400-nm laser fields. On the other hand, the Isat values of different molecules are quite different. In the 800-nm and 400-nm laser fields, the ratios Isat/IADK are both larger than 1, differing from a factor as much as 5.7. Such an observation apparently shows that the molecular Isat is much larger than that of the corresponding atom with the same Ip. This indicates that it is more difficult to ionize the aromatic molecules than the corresponding atoms in strong laser fields, which has also been observed in strong field ionization of many organic molecules.[28] Among the three molecules, chlorobenzene exhibits the largest Isat values in both near-IR and UV laser fields. The difference in strong field ionization among the titled molecules with similar Ip values may be attributed to the different molecular structures and electron orbitals. The physical mechanism of the structure effect could be further revealed if pre-aligned molecules are used in the experiment.
In this work, we investigate and compare the ionizations and fragmentations of benzene, methylbenzene, and chlorobenzene in linearly polarized 800-nm and 400-nm strong laser fields. In low laser intensity where multiphoton ionization is dominant, the parent ions are dominant for benzene and methylbenzene irradiated by the 800-nm laser field, while extensive fragmentation is observed in the 400-nm laser field, which may be understood by the resonant photon absorption of the molecular cations. For chlorobenzene, more significant fragmentations are observed than for benzene and methylbenzene, which may be attributed to the low dissociation energy with chlorine substituent. As the laser intensity is increased, the fragmentations of the molecules are increased for all the molecules, both in the 800-nm laser field and in the 400-nm field. Possible mechanisms for such phenomena are discussed. The saturation intensity of ionization for the titled molecules is measured, and the results indicate that it is more difficult to ionize the aromatic molecules than the corresponding atoms in strong laser fields. Further studies of the ionization and fragmentation of the aromatic molecules in the tunneling ionization regime will be conducted.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|